Answer:
(i) Greater the surface area, greater the extent of adsorption.
If adsorbent is the form of powder form, free valencies are increased and thus
adsorption also increases. [1]
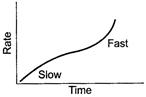
(ii)![]() Ester
hydrolysis is catalysed by acid. In the beginning no external acid (as
catalyst) is not taken. As hydrolysis takes place, reaction is slow. But after sometime
when
Ester
hydrolysis is catalysed by acid. In the beginning no external acid (as
catalyst) is not taken. As hydrolysis takes place, reaction is slow. But after sometime
when ![]() (carboxylic
acid) is formed, it starts working as catalyst and rate is increased. [1]
(carboxylic
acid) is formed, it starts working as catalyst and rate is increased. [1]
 (iii)
(iii) ![]() is
positively charged colloidal sol due to adsorption of
is
positively charged colloidal sol due to adsorption of ![]() on it.
on it. ![]() is
negatively charged colloidal sol due to adsorption of
is
negatively charged colloidal sol due to adsorption of ![]() on it.
on it.
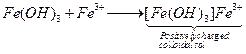
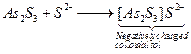 When these two sols are mixed, coagulation takes place
When these two sols are mixed, coagulation takes place
![]()
![]() [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
