Answer:
Colligative properties depend on the number of ions in
solution.
(a) If there is ionisation, number of ions increases hence
colligative properties also increase.
(b) If there is association, number of ions decreases and
in this case also colligative properties decrease.
van't Hoff factor i = [1 + (y -1) x] where, y = number of
ions or molecules from 1 mole solute.
x = degree of ionization or association.
Also![]()
![]()
Molar mass of NaCI determined using depression in freezing point method
Example
Equilibrium
i
Abnormal molecular weight
(i)
Glucose
No change
1
Normal value
(ii)
NaCl
![]()
![]()
( 1 + x )
![]()
(iii)
![]()
![]()
![]()
(1+2x)
![]()
(iv)
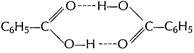
Benzoic acid
![]()
![]() (dimer)
(dimer)
![]()
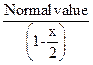
![]() Molar
mass of benzoic acid in benzene solvent using the above method
Molar
mass of benzoic acid in benzene solvent using the above method ![]() as it
forms dimer by H-bonding [2]
as it
forms dimer by H-bonding [2]
You need to login to perform this action.
You will be redirected in
3 sec
