Answer:
(a)
Oxidizing power of halogens: A halogen of lower atomic number will oxidize
halide ion of higher atomic number and therefore will liberate
from their salt solution.
![]() (b) As the size of halogen increases the inter nuclear
forces decreases, thus the acidity increases.
(b) As the size of halogen increases the inter nuclear
forces decreases, thus the acidity increases.
![]() (c) This can be explained on the basis of Lowry
Bronsted concept. According to this concept, a strong acid has a weak conjugate
base and weak acid has a strong conjugate base. The conjugate basis of the
oxyacids of chlorine is shown below:
(c) This can be explained on the basis of Lowry
Bronsted concept. According to this concept, a strong acid has a weak conjugate
base and weak acid has a strong conjugate base. The conjugate basis of the
oxyacids of chlorine is shown below: ![]()
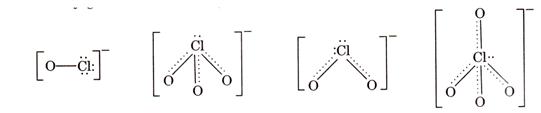 Let us consider the stabilities of the conjugate bases,
Let us consider the stabilities of the conjugate bases, ![]() formed from
these acids
formed from
these acids![]() .
.
![]()
You need to login to perform this action.
You will be redirected in
3 sec
