Answer:
When
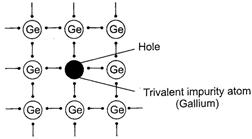
germanium is doped with gallium which contains only three valence electrons
(Group 13 element ns2 np1) the do pant atoms
occupy some of the lattice sites normally occupied by germanium atom. The place
where the fourth valence electron is missing is called electron hole or
electron vacancy.
These holes
can move through the crystal like a positive charge and increase the
conductivity of germanium crystal. It is called p-type semiconductor.
You need to login to perform this action.
You will be redirected in
3 sec
