A) Lone pair- lone pair repuilsion and lone pair-bond pair repulsion
B) Lone pair- lone pair repulsion only
C) Lone pair- bond pair repulsion only
D) Bond pair- bond pair repulsion only
Correct Answer: B
Solution :
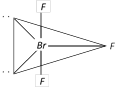 Bent T-shaped geometry in which both lone pairs occupy the equatorial position of the trigonal bipyramidal here \[({{l}_{p}}-{{l}_{p}})\]repulsion = 0\[({{l}_{p}}-{{b}_{p}})\] repulsion = 4 and \[({{b}_{p}}-{{b}_{p}})\] repulsion =2
Bent T-shaped geometry in which both lone pairs occupy the equatorial position of the trigonal bipyramidal here \[({{l}_{p}}-{{l}_{p}})\]repulsion = 0\[({{l}_{p}}-{{b}_{p}})\] repulsion = 4 and \[({{b}_{p}}-{{b}_{p}})\] repulsion =2
You need to login to perform this action.
You will be redirected in
3 sec
