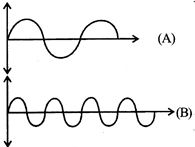
| (i) Velocity |
| (ii) Wavelength |
| (iii) Frequency |
| (iv) Energy |
A) (ii) only
B) (ii) and (iv)
C) (ii), (iii) and (iv)
D) (iv) only
Correct Answer: C
Solution :
[c] e/m waves shown in figure A has higher wavelength in comparison to e/m waves shown in figure B. Thus these waves also differ in frequency and energy, \[v=\frac{v}{\lambda }\]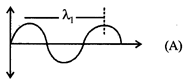 \[\Rightarrow {{E}_{1}}=\frac{hc}{{{\lambda }_{1}}}\]
\[\Rightarrow {{E}_{1}}=\frac{hc}{{{\lambda }_{1}}}\] 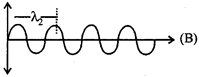 \[\Rightarrow {{E}_{2}}=\frac{hc}{{{\lambda }_{2}}}{{\lambda }_{1}}>{{\lambda }_{2}}\Rightarrow {{E}_{1}}<{{E}_{2}}\]
\[\Rightarrow {{E}_{2}}=\frac{hc}{{{\lambda }_{2}}}{{\lambda }_{1}}>{{\lambda }_{2}}\Rightarrow {{E}_{1}}<{{E}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
