| Read the assertion and reason carefully to mark the correct option out of the options given below: |
| Assertion: The presence of \[A{{g}^{+}}\] enhances the solubility of alkenes in water. |
| Reason: Alkenes are weakly polar in nature. |
A) If both assertion and reason are true and the reason is the correct explanation of the assertion.
B) If both assertion and reason are true but reason is not the correct explanation of the assertion.
C) If assertion is true but reason is false.
D) If the assertion and reason both are false.
E) If assertion is false but reason is true.
Correct Answer: B
Solution :
[b] \[A{{g}^{+}}\] coordinates with the alkene by p\[\pi \]?d\[\pi \] bonding giving an ion and the solubility increases.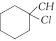
You need to login to perform this action.
You will be redirected in
3 sec
