A) \[Xe{{F}_{2}}\]
B) \[Xe{{O}_{3}}F\]
C) \[Xe{{O}_{2}}{{F}_{2}}\]
D) \[Xe{{F}_{4}}\]
Correct Answer: D
Solution :
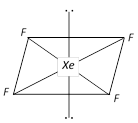 In the formation of \[Xe{{F}_{4}},s{{p}^{3}}{{d}^{2}}\] hybridisation occurs which gives the molecule an octahedral structure. The xenon and four fluorine atoms are coplanar while the two equatorial positions are occupied by the two lone pairs of electrons.
In the formation of \[Xe{{F}_{4}},s{{p}^{3}}{{d}^{2}}\] hybridisation occurs which gives the molecule an octahedral structure. The xenon and four fluorine atoms are coplanar while the two equatorial positions are occupied by the two lone pairs of electrons.
You need to login to perform this action.
You will be redirected in
3 sec
