A) \[{{(\sigma 2{{p}_{x}})}^{1}}\] and \[{{({{\sigma }^{*}}2{{p}_{x}})}^{1}}\]
B) \[{{(\sigma 2{{p}_{x}})}^{1}}\]and \[{{(\pi 2{{p}_{y}})}^{1}}\]
C) \[{{(\pi *2{{p}_{y}})}^{1}}\] and \[{{(\pi *2{{p}_{z}})}^{1}}\]
D) \[{{(\pi *2{{p}_{y}})}^{1}}\]and \[{{(\pi 2{{p}_{y}})}^{1}}\]
E) \[{{(\pi *2{{p}_{z}})}^{1}}\] and \[{{(\pi 2{{p}_{z}})}^{1}}\]
Correct Answer: C
Solution :
The paramagnetic property in oxygen came through unpaired electron which can be explained by molecular orbital theory.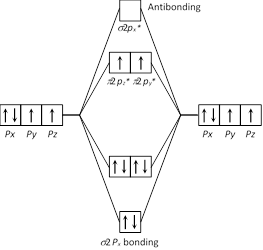 So 2 unpaired of electron present in \[\pi \ 2p_{y}^{*}\ \text{and}\ \pi \ 2p_{z}^{*}\].
So 2 unpaired of electron present in \[\pi \ 2p_{y}^{*}\ \text{and}\ \pi \ 2p_{z}^{*}\].
You need to login to perform this action.
You will be redirected in
3 sec
