A) \[Ni{{\left( CO \right)}_{4}}\] and \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\]are diamagnetic and \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] is paramagnetic
B) \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\] and \[{{\left[ Ni{{\left( CN \right)}_{4}} \right]}^{2-}}\] are diamagnetic and \[Ni{{\left( CO \right)}_{4}}\] is paramagnetic
C) \[Ni{{\left( CO \right)}_{4}}\] and \[{{\left[ Ni{{\left( CN \right)}_{4}} \right]}^{2-}}\] are diamagnetic and \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\] is paramagnetic
D) \[Ni{{\left( CO \right)}_{4}}\] is diamagnetic and \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\] and \[{{\left[ Ni{{\left( CN \right)}_{4}} \right]}^{2-}}\] are paramagnetic
Correct Answer: C
Solution :
The electronic configuration of Ni in \[{{[Ni{{(CN)}_{4}}]}^{2-}},\,{{[Ni(C{{l}_{4}})]}^{2-}}\] and \[Ni{{(CO)}_{4}}\] are as following \[N{{i}^{+}}\]in\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]-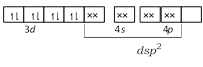 \[N{{i}^{2+}}\]in\[{{[Ni(C{{l}_{4}})]}^{2-}}\]-
\[N{{i}^{2+}}\]in\[{{[Ni(C{{l}_{4}})]}^{2-}}\]- 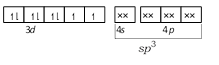 \[Ni\] in \[[Ni{{(CO)}_{4}}]\] -
\[Ni\] in \[[Ni{{(CO)}_{4}}]\] - 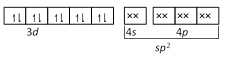 CO and \[C{{N}^{-}}\] are strong ligands so they induces pairing of electrons so their complexes are diamagnetic while \[C{{l}^{-}}\] is a weak ligand so it does not induce the pairing of electrons so its complex is paramagnetic.
CO and \[C{{N}^{-}}\] are strong ligands so they induces pairing of electrons so their complexes are diamagnetic while \[C{{l}^{-}}\] is a weak ligand so it does not induce the pairing of electrons so its complex is paramagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
