A) Three centre bond
B) Hydrogen bond
C) Two centre bond
D) None of the above
Correct Answer: A
Solution :
The diborane molecule has two types of B ? H bond : (i) \[B-{{H}_{t}}\]? It is a normal covalent bond. (ii) \[B-{{H}_{b}}\]? It is a three centred bond.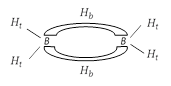
You need to login to perform this action.
You will be redirected in
3 sec
