A)
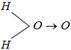
B) \[H-O-O-H\] (straight line)
C)
![]() Where \[\angle H-O-O=\angle O-O-H'={{101.5}^{o}}\]and all the four atoms are in the same plane
Where \[\angle H-O-O=\angle O-O-H'={{101.5}^{o}}\]and all the four atoms are in the same plane
D)
![]() Where \[\angle H-O-O=\angle O-O-H'={{97}^{o}}\] and the angle between \[H-O-O\] plane and \[O-O-H'\] plane is \[{{101}^{o}}\]
Where \[\angle H-O-O=\angle O-O-H'={{97}^{o}}\] and the angle between \[H-O-O\] plane and \[O-O-H'\] plane is \[{{101}^{o}}\]
Correct Answer: D
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
