A) \[s{{p}^{3}}\]
B) \[ds{{p}^{3}}\]
C) \[s{{p}^{3}}{{d}^{2}}\]
D) \[{{d}^{2}}s{{p}^{3}}\]
Correct Answer: D
Solution :
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\] Electronic configuration of \[Fe=[Ar]4{{s}^{2}}3{{d}^{6}}\] Electronic configuration of \[F{{e}^{+3}}=[Ar]3{{d}^{5}}\] Number of ligand (coordination numbr)=6 Nature of ligand is strong field.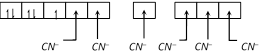 Hybridization of \[Fe\] is \[{{d}^{2}}s{{p}^{3}}\].
Hybridization of \[Fe\] is \[{{d}^{2}}s{{p}^{3}}\].
You need to login to perform this action.
You will be redirected in
3 sec
