A) \[Cl-C{{H}_{2}}-C{{H}_{2}}-OH\]
B)
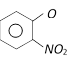
C)
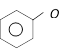
D)
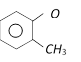
Correct Answer: B
Solution :
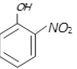 Electron withdrawing group increases acidic character due to -I and -R effect of \[N{{O}_{2}}\] hence orthonitrophenol is most acidic.
Electron withdrawing group increases acidic character due to -I and -R effect of \[N{{O}_{2}}\] hence orthonitrophenol is most acidic.
You need to login to perform this action.
You will be redirected in
3 sec
