A) \[FeC{{l}_{2}}\] and \[CuC{{l}_{2}}\]
B) \[VOC{{l}_{2}}\,\text{and }CuC{{l}_{2}}\]
C) \[VOC{{l}_{2}}\text{ and }FeC{{l}_{2}}\]
D) \[FeC{{l}_{2}}\text{ and }MnC{{l}_{2}}\]
Correct Answer: B
Solution :
Colour of transition metal ion salt is due to \[d-d\] transition of unpaired electrons of d-orbital. Metal ion salt having similar number of similar number of unpaired electron in d-orbitals shows similar colour in aqueous medium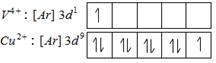 Number of unpaired electrons = 1
Number of unpaired electrons = 1
You need to login to perform this action.
You will be redirected in
3 sec
