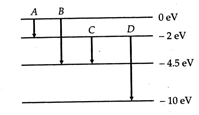
 (b) Which transition corresponds to emission of radiation of (i) maximum wavelength and (ii) minimum wavelength?
(b) Which transition corresponds to emission of radiation of (i) maximum wavelength and (ii) minimum wavelength?
Answer:
(a) The energy \[E\] of a photon of wavelength 275 nm is given by \[E=\frac{hc}{\lambda }\] \[=\frac{6.626\times {{10}^{-34}}\times 3\times {{10}^{8}}}{275\times {{10}^{-9}}}J\] \[=\frac{6.626\times {{10}^{-34}}\times 3\times {{10}^{8}}}{275\times {{10}^{-9}}\times 16\times {{10}^{-19}}}eV\] \[=4.5eV\] This energy corresponds to the transition B for which the energy \[=0-(-45)=45eV.\] (b) Energy of emitted photon, \[E=\frac{hc}{\lambda }\propto \frac{1}{\lambda }\] \[\therefore \] \[{{\lambda }_{\max }}\propto \frac{1}{{{E}_{\min }}}\] and \[{{\lambda }_{\min }}\propto \frac{1}{{{E}_{\max }}}\] (i) Transition A, for which the energy emission is minimum, corresponds to the emission of radiation of maximum wavelength. (ii)Transition D, for which the energy emission is maximum, corresponds to the emission of radiation of minimum wavelength.
You need to login to perform this action.
You will be redirected in
3 sec
