Answer:
(a) According to Bohr's quantisation condition, \[L=m\upsilon {{r}_{n}}=\frac{nh}{2\pi },n=1,2,3...\] or \[2\pi {{r}_{n}}=n\frac{h}{m\upsilon }\] But \[\frac{h}{m\upsilon }=\]de- Broglie wavelength \[(\lambda )\] \[\therefore \] \[2\pi {{r}_{n}}=n\lambda \] Thus the circumference of \[nth\]orbit contains exactly n de- Broglie wavelengths. (b) For third excited state, \[n=4\] For ground state, \[n=1\] Hence, the possible transitions are \[{{n}_{i}}=4\] to \[{{n}_{f}}=3,2,1\] \[{{n}_{i}}=3\]to \[{{n}_{f}}=2,1\] \[{{n}_{i}}=2\] to \[{{n}_{f}}=1\] \[\therefore \]Total number of transitions \[=6,\]as shown in Fig. 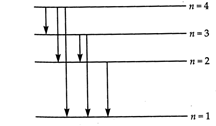
You need to login to perform this action.
You will be redirected in
3 sec
