A) Ionic nature
B) Acidic nature
C) Covalent nature
D) Electron deficient nature
Correct Answer: D
Solution :
Boron halides behave as Lewis acid because of their electron deficient nature eg., as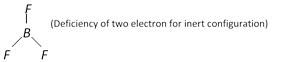
You need to login to perform this action.
You will be redirected in
3 sec
