Answer:
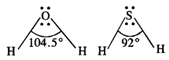
Bond angle in \[{{H}_{2}}O(104\cdot
5{}^\circ )\] is higher than the bond angle in\[{{H}_{2}}S(92{}^\circ )\]. The
difference in the bond angles is linked with the electro negativity's of the
oxygen and sulphur atoms . Since oxygen is more electronegative than sulphur
therefore, the electron density around oxygen m \[{{H}_{2}}O\] is more than
around sulphur in \[{{H}_{2}}S\]. As a result, the bond angle in \[{{H}_{2}}O\]is
more than the bond angle in \[{{H}_{2}}S\]
You need to login to perform this action.
You will be redirected in
3 sec
