Answer:
The molecules of acetone and
chloroform will get linked by intermolecular hydrogen bonding. This means that
the volume of the solution will be less than for ideal solution.
Therefore, \[\Delta {{V}_{(mix)}}\] will
be negative and solution will show negative deviation as compared to ideal
solution.
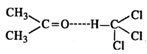
You need to login to perform this action.
You will be redirected in
3 sec
