 (b)
(b)
 (c)
(c)
 (d)
(d)
Answer:
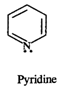
(a) In pyridine (a), the lone electron pair on nitrogen atom is free and is not involved in aromaticity.
(b) In pyrrole (b), the lone electron pair is a part of the aromatic sextet.
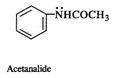
(c) In acetanilide (c), the lone electron pair is involved in conjugation with the \[\pi \]-electron pairs of the ring as well as thecarbonyl group. It is not easily available.
(d) In compound (d), the electron pair is not readily available as it is involved in configuration with the \[\pi \]-electrons of the ring.
In the light of above discussion, in pyridine a nitrogen atom is maximum nucleophilic as well as basic in nature.
You need to login to perform this action.
You will be redirected in
3 sec
