Answer:
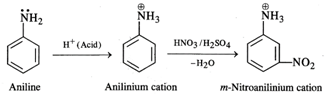
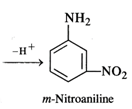
Nitration of aniline with a nitrating
mixture consisting of conc. \[HN{{O}_{3}}\] and conc. \[{{H}_{2}}S{{O}_{4}}\]gives m-nitroaniline as the
product in very small proportions. A major portion of aniline is converted into
a black tarry mass due to partialoxidation of the ring. Under strongly acidic
conditions, aniline is protonated to form a cation which deactivates the ortho
and para positions in the ring. The nitration occurs at the meta position.
You need to login to perform this action.
You will be redirected in
3 sec
