Answer:
(a) Cyanide ion is an ambident ion. It has the following two resonating structures:
![]() Since KCN is predominantly ionic, the reaction can occur either through carbon or nitrogen. Because C-C bonds are stronger than C-N bonds, therefore, carbon end of \[C{{N}^{-}}\] ion acts as the nucleophile on chloromethane to give enthanenitrite as the main product.
Since KCN is predominantly ionic, the reaction can occur either through carbon or nitrogen. Because C-C bonds are stronger than C-N bonds, therefore, carbon end of \[C{{N}^{-}}\] ion acts as the nucleophile on chloromethane to give enthanenitrite as the main product.
![]() In contrast, Ag?CN: is predominantly covalent. Therefore, only electron pair on nitrogen is available for nucleophilic attack on chloromethane to form methyl isocyanide as the chief product
In contrast, Ag?CN: is predominantly covalent. Therefore, only electron pair on nitrogen is available for nucleophilic attack on chloromethane to form methyl isocyanide as the chief product
![]() (b) Due to chemical stability of DDT and its fat solubility, DDT is not completely metabolized very rapidly by the animals. Instead it gets deposited and stored in fatty tissues of animals which causes toxicity. As a result, use of DDT was banned in U.S. in 1973.
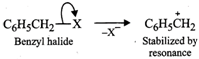
(c) Benzyl halides reality undergo ionization to give benzyl cation which is stabilized by resonance (refer to page 10/31).
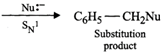
Since carbocations are reactive chemical species, therefore, benzyl halides are very reactive in \[{{S}_{N}}1\] reactions.
(b) Due to chemical stability of DDT and its fat solubility, DDT is not completely metabolized very rapidly by the animals. Instead it gets deposited and stored in fatty tissues of animals which causes toxicity. As a result, use of DDT was banned in U.S. in 1973.
(c) Benzyl halides reality undergo ionization to give benzyl cation which is stabilized by resonance (refer to page 10/31).
Since carbocations are reactive chemical species, therefore, benzyl halides are very reactive in \[{{S}_{N}}1\] reactions.
You need to login to perform this action.
You will be redirected in
3 sec
