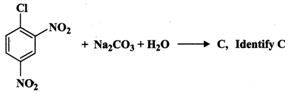 (ii) Predict the product of the reaction.
(ii) Predict the product of the reaction. 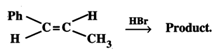
Answer:
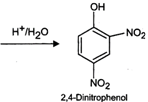
(i) An aqueous solution of \[N{{a}_{2}}C{{O}_{3}}\] is a source of \[O{{H}^{-}}\] ions, i.e.,
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\xrightarrow{\,}\,2N{{a}^{+}}+HCO_{3}^{-}+O{{H}^{-}}\]
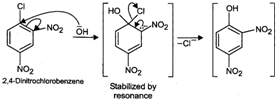
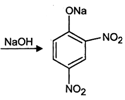
Now \[Cl\] in 2, 4-dinitrochlorobenzene is activated by the presence of two strong electron-withdrawing\[-N{{O}_{2}}\] groups at o-, and p-positions. Therefore, it readily undergoes nucleophilic aromatic substitution to give 2, 4-dinitrophenol after acidification.
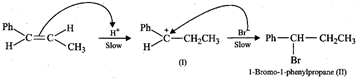
 (ii) Electrophilic addition reaction occurs. In the first step, \[{{H}^{+}}\] adds to that carbon atom of the double bond which carries the \[C{{H}_{3}}\] group since it produces carbocation \[(I)\] which is stabilized by +R-effect of the Ph group and \[+I\]-effect of the \[C{{H}_{2}}C{{H}_{3}}\] group. Subsequent nucleophilic attack by \[B{{r}^{-}}\] on carbocation \[(I)\] gives 1-bromo-1-phenylpropane \[(II)\] as the final product.
(ii) Electrophilic addition reaction occurs. In the first step, \[{{H}^{+}}\] adds to that carbon atom of the double bond which carries the \[C{{H}_{3}}\] group since it produces carbocation \[(I)\] which is stabilized by +R-effect of the Ph group and \[+I\]-effect of the \[C{{H}_{2}}C{{H}_{3}}\] group. Subsequent nucleophilic attack by \[B{{r}^{-}}\] on carbocation \[(I)\] gives 1-bromo-1-phenylpropane \[(II)\] as the final product.
You need to login to perform this action.
You will be redirected in
3 sec
