Answer:
In o- or p-aminobenzoic acids, the
lone pair of electrons on the \[N{{H}_{2}}\] group is donated towards the
benzene ring. As a result, acidic character of\[-COOH\] group and basic
character of \[-N{{H}_{2}}\] group decreases. Therefore, the weakly acidic \[-COOH\]
group cannot transfer a \[{{H}^{+}}\]ion to the weakly basic \[-N{{H}_{2}}\]
group. Thus, o- or p-aminobenzoic acids do not exist as zwitterions.
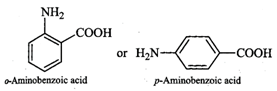
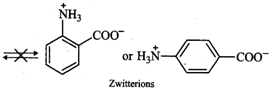 However, in glycine, no such
electron withdrawing benzene ring is present. As a result, \[-N{{H}_{2}}\]
group is sufficiently basic and hence accepts a proton form \[-COOH\] group to
form a zwitterion.
\[\underset{\text{Glycine}}{\mathop{{{H}_{2}}N-C{{H}_{2}}-COOH}}\,\,\rightleftarrows
\,\underset{\text{Zwitterion}}{\mathop{{{H}_{3}}\overset{+}{\mathop{N}}\,-C{{H}_{2}}-CO{{O}^{-}}}}\,\]
However, in glycine, no such
electron withdrawing benzene ring is present. As a result, \[-N{{H}_{2}}\]
group is sufficiently basic and hence accepts a proton form \[-COOH\] group to
form a zwitterion.
\[\underset{\text{Glycine}}{\mathop{{{H}_{2}}N-C{{H}_{2}}-COOH}}\,\,\rightleftarrows
\,\underset{\text{Zwitterion}}{\mathop{{{H}_{3}}\overset{+}{\mathop{N}}\,-C{{H}_{2}}-CO{{O}^{-}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
