Answer:
Due to the presence of lone pairs of electrons on the oxygen atom of the OH group, the carboxylic acids may be regarded as resonance hybrid of structures (\[I\] and \[II\]) :
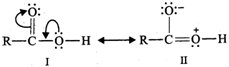 Similarly, a carbonyl group of aldehydes and ketones may be regarded as a resonance hybrid of structures (\[III\] and \[IV\]) :
Similarly, a carbonyl group of aldehydes and ketones may be regarded as a resonance hybrid of structures (\[III\] and \[IV\]) :
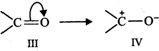 Due to contribution of structure (IV), the carbonyl carbon in aldehydes and ketones is electrophilic. However, due to contribution of structure \[(II)\], the electrophilic character of carboxyl carbon is reduced. In other words, carbonyl carbon of carboxyl group is less electrophilic than carbonyl carbon in aldehydes and ketones and hence nucleophilic addition reactions (such as formation of oximes, hydrazones, phenyl hydrazones, 2, 4-dinitrophenylhydrazones and semicarbazones) of aldehydes and ketones do to take place with carboxylic acids.
Due to contribution of structure (IV), the carbonyl carbon in aldehydes and ketones is electrophilic. However, due to contribution of structure \[(II)\], the electrophilic character of carboxyl carbon is reduced. In other words, carbonyl carbon of carboxyl group is less electrophilic than carbonyl carbon in aldehydes and ketones and hence nucleophilic addition reactions (such as formation of oximes, hydrazones, phenyl hydrazones, 2, 4-dinitrophenylhydrazones and semicarbazones) of aldehydes and ketones do to take place with carboxylic acids.
You need to login to perform this action.
You will be redirected in
3 sec
