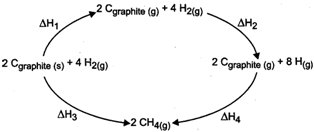
 Find out
(i) Heat of formation of \[C{{H}_{4}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
(ii) Heat of sublimation of \[{{C}_{graphite}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
(iii) Heat of dissociation of \[{{H}_{2}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
Find out
(i) Heat of formation of \[C{{H}_{4}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
(ii) Heat of sublimation of \[{{C}_{graphite}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
(iii) Heat of dissociation of \[{{H}_{2}}\] in terms of \[\Delta {{H}_{1}},\,\Delta {{H}_{2}}\] etc.
Answer:
From the given diagram,
(i) Heat of formation of \[C{{H}_{4}}=\frac{\Delta {{H}_{3}}}{2}\,\] or \[=\frac{\Delta {{H}_{1}}+\Delta {{H}_{2}}+\Delta {{H}_{4}}}{2}\]
(ii) Heat of sublimation of \[{{C}_{graphite}}\,i.e.,\,\,{{C}_{graphite\,(s)}}\,\xrightarrow{\,}\,{{C}_{graphite\,(g)}}=\frac{\Delta {{H}_{1}}}{2}\]
(iii) Heat of dissociation of \[{{H}_{2}},\,i.e.,\,{{H}_{2}}(g)\,\to 2\,H(g)\,=\frac{\Delta {{H}_{2}}}{4}\]
You need to login to perform this action.
You will be redirected in
3 sec
