Answer:
(i) Due to inert pair effect, gallium shows both +1 and +3 oxidation states but its +3 oxidation state is more stable than +1 oxidation state. In other words, +1 gallium is less stable than +3 gallium and hence undergoes disproportionation (self oxidation-reduction) to form gallium metal and the more stable +3 gallium ions in aqueous solution as shown below:
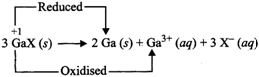 or \[3\,G{{a}^{+}}\,(aq)\xrightarrow{\,}2\,Ga(s)+G{{a}^{3+}}(aq)\]
(ii) Although both \[\text{In}\]and \[\text{Tl}\] can show oxidation states of + 1 and + 3, but inert pair effect is more prominent in \[\text{Tl}\] than in \[\text{In}\]. Therefore, + 1 oxidation state of \[\text{Tl}\] is more stable than its + 3 oxidation state while + 3 oxidation state of \[\text{In}\] is more stable than its + 1 oxidation state. Consequently, in aqueous solution, less stable \[\text{I}{{\text{n}}^{+}}\] undergoes disproportionation to form more state \[\text{I}{{\text{n}}^{3+}}\] but + 1 thallium being more stable does not undergo disproportionation to form + 3 thallium.
or \[3\,G{{a}^{+}}\,(aq)\xrightarrow{\,}2\,Ga(s)+G{{a}^{3+}}(aq)\]
(ii) Although both \[\text{In}\]and \[\text{Tl}\] can show oxidation states of + 1 and + 3, but inert pair effect is more prominent in \[\text{Tl}\] than in \[\text{In}\]. Therefore, + 1 oxidation state of \[\text{Tl}\] is more stable than its + 3 oxidation state while + 3 oxidation state of \[\text{In}\] is more stable than its + 1 oxidation state. Consequently, in aqueous solution, less stable \[\text{I}{{\text{n}}^{+}}\] undergoes disproportionation to form more state \[\text{I}{{\text{n}}^{3+}}\] but + 1 thallium being more stable does not undergo disproportionation to form + 3 thallium.
![]() (iii) As stated above, + 3 oxidation state of \[\text{In}\] is more stable than its + 1 oxidation state, therefore, \[\text{InCl}\]undergoes disproportionation in aqueous solution.
\[\text{3}\,\text{InCl}\,\text{(aq)}\,\xrightarrow{\,}\,2\,\text{I}n\,(s)+\,\text{I}{{n}^{3+}}\,(aq)\]\[+3\,\text{C}{{\text{l}}^{-}}\,(aq)\]
Since +1 oxidation state of \[\text{Tl}\] is more stable than its +3 oxidation state, therefore, \[\text{TlCl}\]does not undergo disproportionation in aqueous solution.
(iii) As stated above, + 3 oxidation state of \[\text{In}\] is more stable than its + 1 oxidation state, therefore, \[\text{InCl}\]undergoes disproportionation in aqueous solution.
\[\text{3}\,\text{InCl}\,\text{(aq)}\,\xrightarrow{\,}\,2\,\text{I}n\,(s)+\,\text{I}{{n}^{3+}}\,(aq)\]\[+3\,\text{C}{{\text{l}}^{-}}\,(aq)\]
Since +1 oxidation state of \[\text{Tl}\] is more stable than its +3 oxidation state, therefore, \[\text{TlCl}\]does not undergo disproportionation in aqueous solution. ![]() (iv) Same as explained in answer (ii) above.
(iv) Same as explained in answer (ii) above.
You need to login to perform this action.
You will be redirected in
3 sec
