Answer:
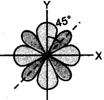
(i) Both have identical shape, consisting of four lobes. (ii) Lobes of \[{{d}_{{{x}^{2}}-{{y}^{2}}}}\] lie along the \[x\] and \[y\]-axes while those of \[{{d}_{xy}}\] lie between \[x\] and \[y\]-axes. (iii) \[45{}^\circ \].
You need to login to perform this action.
You will be redirected in
3 sec
