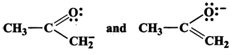 (c)
(c) Answer:
(a) The two structures differ in the position of atoms and hence they are not resonance structures. In fact, these are functional isomers, i.e., \[C{{H}_{3}}-N{{O}_{2}}\] having nitro \[(-N{{O}_{2}})\] group as the functional group and\[C{{H}_{3}}-ONO\] having nitrite \[(O=N-{{O}^{-}})\] as the functional group.
(b)
 These two are resonance structures because they differ in the position of electrons only and not atoms.
(c) These are not resonance structures since they differ in the position of atoms. They are, in fact, tautomers.
These two are resonance structures because they differ in the position of electrons only and not atoms.
(c) These are not resonance structures since they differ in the position of atoms. They are, in fact, tautomers. 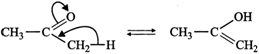 (d) These are not resonance structures since these differ in the position of double bond. In fact, these are position isomers.
(d) These are not resonance structures since these differ in the position of double bond. In fact, these are position isomers.
You need to login to perform this action.
You will be redirected in
3 sec
