Answer:
(i) 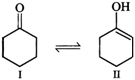
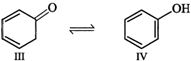 Enol form \[(II)\] is not stabilized and hence it exists in the keto form \[(I)\]
Enol form (IV) is stabilized by resonance energy \[(150.6\,kJ\,mo{{l}^{-1}})\] of the benzene ring and hence \[(III)\] exists in the enol form (IV)
(ii) In \[{{H}_{2}}C=C{{H}^{-}},\] the carbon atom carrying the -ve charge is \[s{{p}^{2}}\]-hybridized while in \[HC\equiv {{C}^{-}},\] the carbon atom carrying the -ve charge is sp-hybridized. Since a \[s{{p}^{2}}\]-hybridized carbon is less electronegative than a sp-hybridized carbon, therefore, \[{{H}_{2}}C=C{{H}^{-}}\]is a better nucleophile than \[HC\equiv {{C}^{-}}\].
Enol form \[(II)\] is not stabilized and hence it exists in the keto form \[(I)\]
Enol form (IV) is stabilized by resonance energy \[(150.6\,kJ\,mo{{l}^{-1}})\] of the benzene ring and hence \[(III)\] exists in the enol form (IV)
(ii) In \[{{H}_{2}}C=C{{H}^{-}},\] the carbon atom carrying the -ve charge is \[s{{p}^{2}}\]-hybridized while in \[HC\equiv {{C}^{-}},\] the carbon atom carrying the -ve charge is sp-hybridized. Since a \[s{{p}^{2}}\]-hybridized carbon is less electronegative than a sp-hybridized carbon, therefore, \[{{H}_{2}}C=C{{H}^{-}}\]is a better nucleophile than \[HC\equiv {{C}^{-}}\].
You need to login to perform this action.
You will be redirected in
3 sec
