Answer:
In acetamide, the carbonyl group is electron withdrawing in nature. It decreases the electron density on the nitrogen atom of the amino group due to conjugation. On the other hand, in ethylamine, the ethyl group has \[+I\]
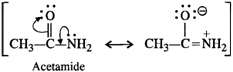 \[\underset{\text{Ethylamine}}{\mathop{{{C}_{2}}{{H}_{5}}\to -\overset{\centerdot \,\,\centerdot }{\mathop{N}}\,{{H}_{2}}}}\,\]
effect and it increases the electron density on the nitrogen atom. Therefore, electron releasing tendency is more in ethylamine than in acetamide or the former is a stronger base.
\[\underset{\text{Ethylamine}}{\mathop{{{C}_{2}}{{H}_{5}}\to -\overset{\centerdot \,\,\centerdot }{\mathop{N}}\,{{H}_{2}}}}\,\]
effect and it increases the electron density on the nitrogen atom. Therefore, electron releasing tendency is more in ethylamine than in acetamide or the former is a stronger base.
You need to login to perform this action.
You will be redirected in
3 sec
