Answer:
The structures of the products of ozonolysis are:
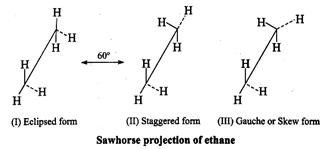 Since, the total number of carbon atoms of the two products is 11 (4 + 7) while the MF of the conjugated diene is \[{{C}_{13}}{{H}_{22}},\] therefore, the ozonolysis must have also produced another two carbon product. Further since the given compound \[({{C}_{13}}{{H}_{22}})\] is an alkadiene, therefore, this two carbon product must be glyoxal, \[O=CH-CH=O\]. Replace the oxygen atoms from these three products by double bonds, the structure of the alkadiene is
Since, the total number of carbon atoms of the two products is 11 (4 + 7) while the MF of the conjugated diene is \[{{C}_{13}}{{H}_{22}},\] therefore, the ozonolysis must have also produced another two carbon product. Further since the given compound \[({{C}_{13}}{{H}_{22}})\] is an alkadiene, therefore, this two carbon product must be glyoxal, \[O=CH-CH=O\]. Replace the oxygen atoms from these three products by double bonds, the structure of the alkadiene is
You need to login to perform this action.
You will be redirected in
3 sec
