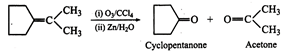
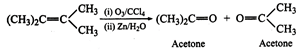
Answer:
In trans-but-2-ene, the dipole
moments of the two \[C-C{{H}_{3}}\]bonds are equal and opposite and hence they exactly
cancel out each other. Thus, trans-but- 2-ene is non-polar
 However, in trans-pent-2-ene,
the\[+I\]-effect of \[C{{H}_{3}}C{{H}_{2}}\]-group is higher than that of \[C{{H}_{3}}\]-group,
therefore, the dipole moments of \[C-C{{H}_{3}}\] and \[C-C{{H}_{2}}C{{H}_{3}}\]
bonds are unequal. Although these two dipoles oppose each other, yet they do
not exactly cancel out each other and hence trans-pent-2-ene has a small but
finite dipole moment and thus is polar.
However, in trans-pent-2-ene,
the\[+I\]-effect of \[C{{H}_{3}}C{{H}_{2}}\]-group is higher than that of \[C{{H}_{3}}\]-group,
therefore, the dipole moments of \[C-C{{H}_{3}}\] and \[C-C{{H}_{2}}C{{H}_{3}}\]
bonds are unequal. Although these two dipoles oppose each other, yet they do
not exactly cancel out each other and hence trans-pent-2-ene has a small but
finite dipole moment and thus is polar.
You need to login to perform this action.
You will be redirected in
3 sec
