Answer:
The valence shell electronic configurations of both N and P are as follows : 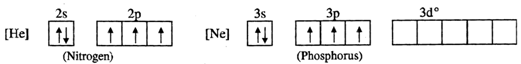 Since nitrogen atom has no vacant 2d orbitals, it cannot extend its covalency to five. Therefore, a molecule of\[NC{{l}_{5}}\]does not exist. But in case of phosphorus, an electron can be promoted from 3s filled orbital to 3d vacant orbitals. Therefore, phosphorus can extend its covalency to five. Thus, a molecule of \[PC{{l}_{5}}\] can exist.
Since nitrogen atom has no vacant 2d orbitals, it cannot extend its covalency to five. Therefore, a molecule of\[NC{{l}_{5}}\]does not exist. But in case of phosphorus, an electron can be promoted from 3s filled orbital to 3d vacant orbitals. Therefore, phosphorus can extend its covalency to five. Thus, a molecule of \[PC{{l}_{5}}\] can exist.
You need to login to perform this action.
You will be redirected in
3 sec
