Answer:
In the vapour state at high temperature, \[BeC{{l}_{2}}\] exists as linear molecule, \[Cl-Be-Cl\]. The hybridization of the central atom is sp. In the solid state, it has a polymeric structure with chlorine bridges as follows:
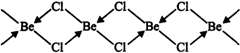 Two \[Cl\] atoms are linked to \[Be\] atom by two coordinate bonds and two by covalent bonds. For these bonds to be formed. Be in the excited state with the configuration \[1{{s}^{2}}2{{s}^{1}}2p_{x}^{1}2p_{y}^{0}2p_{z}^{0}\] undergoes \[s{{p}^{3}}\]hybridisation. Two half-filled hybrid orbitals will form normal covalent bonds with two \[Cl\] atom. The other two \[Cl\] atoms are coordinated to \[Be\] atom by donating electron pairs into the empty hybrid orbitals.
Two \[Cl\] atoms are linked to \[Be\] atom by two coordinate bonds and two by covalent bonds. For these bonds to be formed. Be in the excited state with the configuration \[1{{s}^{2}}2{{s}^{1}}2p_{x}^{1}2p_{y}^{0}2p_{z}^{0}\] undergoes \[s{{p}^{3}}\]hybridisation. Two half-filled hybrid orbitals will form normal covalent bonds with two \[Cl\] atom. The other two \[Cl\] atoms are coordinated to \[Be\] atom by donating electron pairs into the empty hybrid orbitals.
You need to login to perform this action.
You will be redirected in
3 sec
