Answer:
In both the molecules, the oxygen atom is \[s{{p}^{3}}\] hybridised and the expected bond angle is \[109{}^\circ 28'.\]
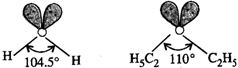 However, the presence of two lone pairs distorts the geometry of both these molecules. The bond angle in diethyl ether molecule is more than in case of water molecule because of greater repulsion in two \[{{C}_{2}}{{H}_{5}}\] groups as compared to H atoms in the molecule of water.
However, the presence of two lone pairs distorts the geometry of both these molecules. The bond angle in diethyl ether molecule is more than in case of water molecule because of greater repulsion in two \[{{C}_{2}}{{H}_{5}}\] groups as compared to H atoms in the molecule of water.
You need to login to perform this action.
You will be redirected in
3 sec
