Some Important Man made Materials
Category : Railways
Introduction
Chemistry has helped significantly in meeting human needs by providing chemical fertilizers, improved varieties of insecticides and pesticides to increase the yield of crops and fruits. It has given us a large number of lifesaving drugs. Also chemical industries manufacturing polymers, soaps, detergents, glass, ceramics etc.
Industrially Important Compounds Glass
It consists of a mixture of two or more silicates.
\[N{{a}_{2}}C{{O}_{3}}(s)+\underset{\left( silica \right)}{\mathop{Si{{O}_{2}}(s)}}\,\to N{{a}_{2}}Si{{O}_{3}}(s)+C{{O}_{2}}(g)\]
\[N{{a}_{2}}S{{O}_{4}}(s)+Si{{O}_{2}}(s)\to N{{a}_{2}}Si{{O}_{3}}(s)+S{{O}_{3}}(g)\]
\[CaC{{O}_{3}}(s)+Si{{O}_{2}}(s)\to CaSi{{O}_{3}}(s)+C{{O}_{2}}(g)\]
(i) It is resistant to action of air and acids except hydroflouric acid.
(ii) It is alkaline in nature.
(iii) It slowly reacts with water to form alkaline solution.
(i) Silica glass: For this type of glass the raw material used is 100% pure form of quartz. It is quite expensive. It is used in the manufacture of laboratory apparatus. It has low thermal expansion. Its softening point is very high and it is resistant to a wide variety of chemicals.
(ii) Alkali silicate glass: For it the raw materials used are sand and soda. It is also called water glass because it is soluble in water and used only as a solution. It is generally used to make gums and adhesives.
(iii) Lead glass: For this type of glass lead oxide is added to ordinary glass. The addition of lead oxide increases the density and also the refractive index. This type of glass is used for the manufacture of ornamental glass ware, decorative articles etc.
(iv) Optical glass: This type of glass is used in the manufacture of optical instruments like binoculars, spectacles, lenses, prisons, telescopes, microscopes etc. It is transparent and can be grounded into the required shape. It generally contains phosphorus, and lead silicates with little cerium oxide which absorbs UV radiations.
(v) Processed glass: The properties and applications of glass also depend upon the processing of glass. Some types of processed glass and their applications are given here:
|
Processed glass |
Applications |
|
1. Laminated glass |
Used for doors and windows of automobiles. (It has high strength). |
|
2. Fibre glas |
Used for reinforcing purpose (It has enough tensile strength) |
|
3. Foam glass |
Used for civil construction and insulation purposes (it is light weight). |
|
4. Opaque glass |
In it non-transparent glass filters the light entering into it. Thus provides an aesthetic look. |
(vi) Borosilicate glass: It contains silica and Boron oxide and small amount of oxides of sodium and aluminium. It is resistant to a wide variety of chemicals due to this property it is used in the manufacture of laboratory ware.
Fertilizers
Fertilizers are chemical compounds which when added to the soil increase their fertility and directly supply the need of essential elements [N, P, K] of primary importance.
Some Important Man Made Materials
(i) Nitrogenous fertilizers: Ammonium sulphate, urea etc.
(ii) Phosphatic fertilizers: Super phosphate, ammonium phosphate
(iii) Potash fertilizers: Potassium chloride, potassium sulphate.
Soaps and detergents
Fatty acid + sodium hydroxide \[\to \] Soap + glycerol.
(i) Detergents can be used for laundering even with hard water as they are soluble even in hard water.
(ii) Detergents possess better cleansing properties than soaps.
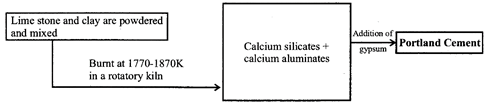
Portland cement
Calcium oxide (CaO) 62%
Silica \[(Si{{O}_{2}})\] 22%
Alumina \[(Si{{O}_{2}})\] 7.5%
Magnesia \[(MgO)\] 2.5%
Ferric oxide \[(F{{e}_{2}}{{O}_{3}})\] 2.5%
The above compounds are provided by the two raw materials, namely lime stone (which provides CaO) and clay (which provides\[Si{{O}_{2}}\], \[A{{l}_{2}}{{O}_{3}}\] and \[F{{e}_{2}}{{O}_{3}}\]. In cement, almost entire amount of lime in present in the combined state as calcium silicate (2CaO. \[Si{{O}_{2}}\] and 3CaO. \[Si{{O}_{2}}\]) and calcium aluminates (3CaO. \[A{{l}_{2}}{{O}_{3}}\] and 4 CaO. \[A{{l}_{2}}{{O}_{3}}\]).
(i) Cement containing excess amount of lime cracks during setting; while cement containing less amount of lime is weak in strength.
(ii) Cement with excess of silica is slow-setting and that having an excess of alumina is quick-setting.
(iii) Cement containing no iron is white but hard to bum. Cement is manufactured by two processes, viz. wet and dry. A small amount (2-3%) of gypsum is added to slow down the setting of the cement so that it gets sufficiently hardened. Setting of cement is an exothermic process and involves hydration of calcium aluminates and calcium silicates.
Vitamins
|
Solubility |
Deficiency disease |
Overdose disease |
|
|
Vitamin A |
Fat |
Nightblindness and Keratomalacia |
Hypervitamino sis |
|
Vitamin \[{{B}_{1}}\] |
Water |
Beriberi, Wemicke-Korsak off syndrome |
Drowsiness of music relaxation with large doses |
|
Vitamin \[{{B}_{2}}\] |
Water |
Ariboflavinosis |
|
|
Vitamin \[{{B}_{3}}\] |
Water |
Pellagra |
Liver damage (doses > 2g/day) and other problems |
|
Vitamin \[{{B}_{5}}\] |
Water |
Paresthesia |
Diarrohea; possibly nausea and heartburn |
|
Vitamin \[{{B}_{6}}\] |
Water |
Anemia peripheral neuropathy nerve damage (dose > 100 mg/day) |
Impairment of proprioception |
|
Vitamin \[{{B}_{7}}\] |
Water |
Dematitis, enteritis |
|
|
Vitamin \[{{B}_{9}}\] |
Water |
Deficiency during pregnancy is deficiency, other effects |
May mask symptoms of \[Vita\min {{e}_{12}}\] associated with birth defects, such as neural tube defects |
|
Vitamin \[{{B}_{12}}\] |
Water |
Megaloblastic anemia |
No known toxicity |
|
Vitamin C |
Water |
Scurvy |
Vitamin C megadosage |
|
Vitamin D |
Fat |
Rickets and Osteomalancia |
Hypeirvitamin osis D |
|
Vitamin E |
Fat |
Deficiency is very rare; mild hemolytic anemia in newbom |
Increased congestive heart failure seen in one large |
|
Vitamin K |
Fat |
Bleeding diathesis |
Increases coagulation in patients taking warfarin. |
You need to login to perform this action.
You will be redirected in
3 sec
