Photo-Electric Effect
Category : JEE Main & Advanced
The photo-electric effect is the emission of electrons (called photo-electrons when light strikes a surface. To escape from the surface, the electron must absorb enough energy from the incident radiation to overcome the attraction of positive ions in the material of the surface. The photoelectric effect was first observed by Heinrich Hertz and it was investigated in detail by Whilelm Hallwachs and Philipp Lenard. The photoelectric effect is based on the principle of conservation of energy.
(1) Work function (or threshold energy) \[({{W}_{0}})\]: The minimum energy of incident radiation, required to eject the electrons from metallic surface is defined as work function of that surface.
\[{{W}_{0}}=h{{\nu }_{0}}=\frac{hc}{{{\lambda }_{0}}}Joules\,;\] \[{{v}_{0}}=\] Threshold frequency;
\[{{\lambda }_{0}}=\]Threshold wavelength
Work function in electron volt \[{{W}_{0}}(eV)\]\[=\frac{hc}{e{{\lambda }_{0}}}=\frac{12375}{{{\lambda }_{0}}({\AA})}\]
Work function of several elements
| Element | Work function (eV) | Element | Work function (eV) |
| Platinum | 6.4 | Aluminum | 4.3 |
| Gold | 5.1 | Silver | 4.3 |
| Nickel | 5.1 | Sodium | 2.7 |
| Carbon | 5.0 | Lithium | 2.5 |
| Silicon | 4.8 | Potassium | 2.2 |
| Copper | 4.7 | Cesium | 1.9 |
(2) Threshold frequency \[\mathbf{(}{{\mathbf{v}}_{\mathbf{0}}}\mathbf{)}\]: The minimum frequency of incident radiations required to eject the electron from metal surface is defined as threshold frequency.
If incident frequency \[v<{{v}_{0}}\Rightarrow \]No photoelectron emission
For most metals the threshold frequency is in the ultraviolet (corresponding to wavelengths between 200 and 300 nm), but for potassium and cesium oxides it is in the visible spectrum (\[\lambda \] between 400 and 700 nm)
(3) Threshold wavelength \[({{\lambda }_{0}})\]: The maximum wavelength of incident radiations required to eject the electrons from a metallic surface is defined as threshold wavelength.
If incident wavelength \[\lambda >{{\lambda }_{0}}\Rightarrow \] No photoelectron emission
(4) Einstein's photoelectric equation : According to Einstein, photoelectric effect is the result of one to one inelastic collision between photon and electron in which photon is completely absorbed
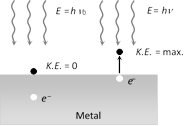
Einstein's photoelectric equation is \[E={{W}_{0}}+{{K}_{\max }}\]
where \[{{K}_{\max }}=\frac{1}{2}mv_{\max }^{2}=\]maximum kinetic energy of emitted electrons.
You need to login to perform this action.
You will be redirected in
3 sec
