Chemical Effect of Current
Category : JEE Main & Advanced
Current can produce or speed up chemical change, this ability of current is called chemical effect (shown by dc not by ac).
(1) Electrolytes : The liquids which allows the current to pass through them and also dissociates into ions on passing current through them are called electrolytes e.g. solutions of salts, acids and bases in water, etc.
Those liquids which do not allow current to pass through them are called insulators (e.g. vegetable oils, distilled water etc.)
Solutions of cane sugar, glycerin, alcohol etc. are examples of non-electrolytes.
(2) Electrolysis : The process of decomposition of electrolyte solution into ions on passing the current through it is called electrolysis.
Practical applications of electrolysis are Electrotyping, extraction of metals from the ores, Purification of metals, Manufacture of chemicals, Production of \[{{O}_{2}}\] and \[{{H}_{2}}\], Medical applications and electroplating.
(3) Electroplating : It is a process of depositing a thin layer of one metal over another metal by the method of electrolysis. The articles of cheap metals are coated with precious metals like silver and gold to make their look more attractive. The article to be electroplated is made the cathode and the metal to be deposited is made the anode. A soluble salt of the precious metal is taken as the electrolyte. (If gold is to be coated then auric chloride is used as electrolyte).
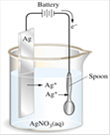
(4) Voltameter : The vessel in which the electrolysis is carried out is called a voltameter. It contains two electrodes and electrolyte. It is also known as electrolytic cell.
Types of voltameters
| Volatameter | Anode/cathode | Electrolyte | Deposition |
|
Cu voltameter
|
Cathode may be of any material but anode must be of Cu | \[CuS{{O}_{4}}\] or \[CuC{{l}_{2}}\] | At cathode Cu deposited |
|
Ag voltameter
|
Cathode may be of any material but anode must be of Ag | \[AgN{{O}_{3}}\] | At cathode Ag deposited |
|
Water voltameter
|
Both electrode are made of platinum (Pt) | Acidulated water | \[{{H}_{2}}\] and \[{{O}_{2}}\] gases are collects over the cathode and anode respectively in the ratio of 2 : 1 |
You need to login to perform this action.
You will be redirected in
3 sec
