Hydrogen Spectrum and Spectral Series
Category : JEE Main & Advanced
When hydrogen atom is excited, it returns to its normal unexcited (or ground state) state by emitting the energy it had absorbed earlier. This energy is given out by the atom in the form of radiations of different wavelengths as the electron jumps down from a higher to a lower orbit. Transition from different orbits cause different wavelengths, these constitute spectral series which are characteristic of the atom emitting them. When observed through a spectroscope, these radiations are imaged as sharp and straight vertical lines of a single colour.

The spectral lines arising from the transition of electron forms a spectra series.
(1) Mainly there are five series and each series is named after it's discover as Lymen series, Balmer series, Paschen series, Bracket series and Pfund series.
(2) According to the Bohr's theory the wavelength of the radiations emitted from hydrogen atom is given by \[\frac{1}{\lambda }=R\,\left[ \frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}} \right]\]\[\Rightarrow \]\[\lambda =\frac{n_{1}^{2}n_{2}^{2}}{(n_{2}^{2}-n_{1}^{2})R}=\frac{n_{1}^{2}}{\left( 1-\frac{n_{1}^{2}}{n_{2}^{2}} \right)R}\]
where \[{{n}_{2}}=\]outer orbit (electron jumps from this orbit), \[{{n}_{1}}=\] inner orbit (electron falls in this orbit)
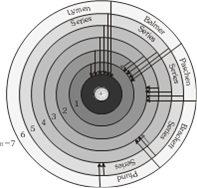
(3) First line of the series is called first member, for this line wavelength is maximum \[({{\lambda }_{\max }})\]
For maximum wavelength if \[{{n}_{1}}=n\] then \[{{n}_{2}}=n+1\]
So \[{{\lambda }_{\max }}=\frac{{{n}^{2}}{{(n+1)}^{2}}}{(2n+1)R}\]
(4) Last line of the series is called series limit, for this line wavelength is minimum \[({{\lambda }_{\min }})\]
For minimum wavelength \[{{n}_{2}}=\infty ,\,{{n}_{1}}=n\] So \[{{\lambda }_{\min }}=\frac{{{n}^{2}}}{R}\]
(5) The ratio of first member and series limit can be calculated as \[\frac{{{\lambda }_{\max }}}{{{\lambda }_{\min }}}=\frac{{{(n+1)}^{2}}}{(2n+1)}\]
Different spectral series
| Spectral series | Transition | \[{{\lambda }_{\max }}\] | \[{{\lambda }_{\min }}\] | \[\frac{{{\lambda }_{max}}}{{{\lambda }_{min}}}\] | Region |
| 1. Lymen series | \[{{n}_{2}}=\] 2, 3, 4 ... \[\infty \] \[{{n}_{1}}=1\] | \[\frac{4}{3R}\] | \[\frac{1}{R}\] | \[\frac{4}{3}\] | Ultraviolet region |
| 2. Balmer series | \[{{n}_{2}}=\] 3, 4, 5 ... \[\infty \] \[{{n}_{1}}=2\] | \[\frac{36}{5R}\] | \[\frac{4}{R}\] | \[\frac{9}{5}\] | Visible region |
| 3. Paschen series | \[{{n}_{2}}=\] 4, 5, 6 ... \[\infty \] \[{{n}_{1}}=3\] | \[\frac{144}{7R}\] | \[\frac{9}{R}\] | \[\frac{16}{7}\] | Infrared region |
| 4. Bracket series | \[{{n}_{2}}=\] 5, 6, 7 ... \[\infty \] \[{{n}_{1}}=4\] | \[\frac{400}{9R}\] | \[\frac{16}{R}\] | \[\frac{25}{9}\] | Infrared region |
| 5. Pfund series | \[{{n}_{2}}=\] 6, 7, 8 ... \[\infty \] \[{{n}_{1}}=5\] | \[\frac{900}{11R}\] | \[\frac{25}{R}\] | \[\frac{36}{11}\] | Infrared region |
You need to login to perform this action.
You will be redirected in
3 sec
