Nitrogen Family
Category : JEE Main & Advanced
Nitrogen is the first member of group 15 or VA of the periodic table. It consists of five elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi). The elements of this group are collectively called pnicogens and their compounds as pniconides. The name is derived from Greek word ?Pniomigs? meaning suffocation. Pniconide contain \[{{M}^{3-}}\] species.
(1) Electronic configuration
| Elements | Electronic configuration (\[n{{s}^{2}}\ n{{p}^{3}}\]) |
| \[_{7}N\] | \[[He]\,2{{s}^{2}}2{{p}^{3}}\] |
| \[_{15}P\] | \[[Ne]\,3{{s}^{2}}3{{p}^{3}}\] |
| \[_{33}As\] | \[[Ar]\,3{{d}^{10}}4{{s}^{2}}4{{p}^{3}}\] |
| \[_{51}Sb\] | \[[Kr]\,4{{d}^{10}}5{{s}^{2}}5{{p}^{3}}\] |
| \[_{83}Bi\] | \[[Xe]\,4{{f}^{14}}5{{d}^{10}}6{{s}^{2}}6{{p}^{3}}\] |
Physical properties
(1) Physical state : Nitrogen– (gas), phosphorus – (solid) (vaporises easily), As, Sb, Bi–solids.
Nitrogen is the most abundant gas in the atmosphere. It constitutes about 78% by volume of the atmosphere. Phosphorus is the most reactive element in this group and its yellow form is always kept under water.
(2) Atomic radii : Atomic radii increases with atomic number down the group i.e., from N to Bi due to addition of extra principal shell in each succeding elements.
(3) Ionisation energy : The ionisation values of the elements of this group decreases down the group due to gradual increases in atomic size.
(4) Electronegativity : Generally the elements of nitrogen family have high value of electronegativity. This value shows a decreasing trend in moving down the group from nitrogen to bismuth.
(5) Non-metallic and metallic character : Nitrogen and phosphorus are non-metals, arsenic and antimony are metalloids (semi-metal) and bismuth a typical metal.
(6) Molecular state : Nitrogen readily forms triple bond (two pp –pp bonds) and exists as discrete diatomic gaseous molecule \[(N\equiv N)\] at room temperature. Phosphorus, arsenic and antimony exist in the form of discrete tetra atomic molecules such as \[{{P}_{4}},A{{s}_{4}},S{{b}_{4}}\] in which the atoms are linked to each other by single bonds.
(7) Melting and boiling points : The melting points and boiling points of group 15 elements do not show a regular trend.
M.pt. first increases from N to As and then decreases from As to Bi. Boiling point increases from N to Sb. Boiling point of Bi is less than Sb.
(8) Allotropy : All the members of group 15 except Bi exhibit the phenomenon of allotropy.
(i) Nitrogen exists in two solid and one gaseous allotropic forms.
(ii) Phosphorus exists in several allotropic forms such as white, red, scarlet, violet and black form.
(a) White or yellow phosphorus : White phosphorus is prepared from rock phosphate \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}},Si{{O}_{2}}\] and coke which are electrically heated in a furnace.
\[2C{{a}_{3}}{{(P{{O}_{4}})}_{2}}+6Si{{O}_{2}}\xrightarrow{\Delta }6CaSi{{O}_{3}}+{{P}_{4}}{{O}_{10}}\];
\[{{P}_{4}}{{O}_{10}}+10C\xrightarrow{\Delta }{{P}_{4}}+10CO\]\[\]
When exposed to light, it acquires a yellow colour.
(b) Red phosphorus : It is obtained by heating yellow phosphorus, between 240 –250°C in the presence of an inert gas. Yellow phosphorus can be separated from red phosphorus by reaction with NaOH (aq) or KOH (aq) when the former reacts and the latter remains unreacted.
(iii) Arsenic exists in three allotropic forms namely grey, yellow and black. Antimony also exists in three forms, viz., metallic, yellow and explosive.
(9) Oxidation state : The members of the group 15 exhibit a number of positive and negative oxidation states.
(i) Positive oxidation states : The electronic configuration \[(n{{s}^{2}}n{{p}^{3}})\]for the valence shell of these elements shows that these elements can have +3 and +5 oxidation states. In moving down this group, the stability of +3 oxidation state increases. It may be pointed out here that nitrogen does not exhibit an oxidation state of +5, because it fails to expand its octet due to nonavailability of vacant d-orbitals.
(ii) Negative oxidation states : For example oxidation state of nitrogen is –3. The tendency of the elements to show –3 oxidation state decreases on moving down the group from N to Bi.
(10) Catenation (self linkage) : Elements of group 15 also show some tendency to exhibit catenation. This tendency goes on decreasing in moving down the group due to gradual decrease in their bond (M–M) energies.
Chemical properties
(1) Hydrides : All the members form volatile hydrides of the type \[A{{H}_{3}}\]. All hydrides are pyramidal in shape. The bond angle decreases on moving down the group due to decrease in bond pair–bond pair repulsion.
\[\underset{{{107}^{o}}}{\mathop{N{{H}_{3}}}}\,\,\,\,\,\,\underset{{{94}^{o}}}{\mathop{P{{H}_{3}}}}\,\,\,\,\,\,\underset{{{92}^{o}}}{\mathop{As{{H}_{3}}}}\,\,\,\,\,\,\underset{{{91}^{o}}}{\mathop{Sb{{H}_{3}}}}\,\,\,\,\,\,\underset{{{90}^{o}}}{\mathop{Bi{{H}_{3}}}}\,\]
The decreasing order of basic strength of hydrides is as follows : \[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}>Bi{{H}_{3}}\].
The increasing order of boiling points is as follows :
\[P{{H}_{3}}<As{{H}_{3}}<N{{H}_{3}}<Sb{{H}_{3}}\].
\[N{{H}_{3}}\] is thermally most stable and \[Bi{{H}_{3}}\] is least stable. This is because in \[N{{H}_{3}}\], N – H covalent bond is the strongest due to small size of N atom. Hence, the decomposition temperature of \[N{{H}_{3}}\] will be the highest. The increasing order of reducing character is as follows, \[N{{H}_{3}}<P{{H}_{3}}<As{{H}_{3}}<Sb{{H}_{3}}<Bi{{H}_{3}}\].
(2) Halides : The members of the family form trihalides \[(M{{X}_{3}})\] and pentahalides \[(M{{X}_{5}})\]. The trihalides are \[s{{p}^{3}}\]-hybridized with distorted tetrahedral geometry and pyramidal shape while pentahalides are \[s{{p}^{3}}d\]-hybridized and are trigonal bipyramidal in shape. The trihalides are hydrolysed by water and ease of hydrolysis decreases when we move down the group. Hence, \[NC{{l}_{3}}\] is easily hydrolysed but \[SbC{{l}_{3}}\] and \[BiC{{l}_{3}}\] are partly and reversibly hydrolysed. \[N{{F}_{3}}\] is not hydrolysed due to lack of vacant d-orbital with nitrogen. \[P{{F}_{3}}\] and \[P{{F}_{5}}\] are also not hydrolysed because the P – F bond is stronger than P – O covalent bond. The hydrolysis products of the halides are as follows :
\[NC{{l}_{3}}+3{{H}_{2}}O\to N{{H}_{3}}+3HOCl\]
\[PC{{l}_{3}}+3{{H}_{2}}O\to {{H}_{3}}P{{O}_{3}}+3HCl\]
\[2AsC{{l}_{3}}+3{{H}_{2}}O\to A{{s}_{2}}{{O}_{3}}+6HCl\]
\[SbC{{l}_{3}}+{{H}_{2}}O\to SbOCl+2HCl\]
\[BiC{{l}_{3}}+{{H}_{2}}O\to BiOCl+2HCl\]
Their basic character follows this decreasing order as \[N{{I}_{3}}>NB{{r}_{3}}>NC{{l}_{3}}>N{{F}_{3}}\]. Except \[N{{F}_{3}}\], the trihalides of nitrogen are unstable and decompose with explosive violence. \[N{{F}_{3}}\] is stable and inert. \[NC{{l}_{3}}\] is highly explosive. Trifluorides and trichlorides of phosphorus and antimony act as Lewis acid. The acid strength decreases down the group. For example, acid strength of tri-chlorides is in the order ; \[PC{{l}_{3}}>AsC{{l}_{3}}>SbC{{l}_{3}}\].
Nitrogen does not form pentahalides due to non-availability of vacant d-orbitals. The pentachloride of phosphorus is not very stable because axial bonds are longer (and hence weaker) than equitorial bond. Hence, \[PC{{l}_{5}}\] decomposes to give \[PC{{l}_{3}}\] and \[C{{l}_{2}}\]; \[PC{{l}_{5}}\] \[\rightleftharpoons \] \[PC{{l}_{3}}+C{{l}_{2}}\].
The unstability of \[PC{{l}_{5}}\] makes it a very good chlorinating agent. All pentahalides act as lewis acids since they can accept a lone pair of electron from halide ion.
Solid \[PC{{l}_{5}}\] is an ionic compound consisting of \[{{[PC{{l}_{4}}]}^{+}}\,\,\,\,{{[PC{{l}_{6}}]}^{-}},\,\,\,\,{{[PC{{l}_{4}}]}^{+}}\] has a tetrahedral structure, while \[{{[PC{{l}_{6}}]}^{-}}\] has an octahedral structure.
Since, \[PC{{l}_{5}}\] reacts readily with moisture it is kept in well stoppered bottles.
\[P{{I}_{5}}\] does not exist due to large size of I atoms and lesser electronegativity difference between phosphorus and iodine.
Down the group, the tendency to form pentahalides decreases due to inert pair effect. e.g., \[Bi{{F}_{5}}\] does not exist.
(3) Oxides : These elements form oxides of the type \[{{X}_{2}}{{O}_{3}},{{X}_{2}}{{O}_{4}}\] and \[{{X}_{2}}{{O}_{5}}\].
The acidic strength of oxides :
\[{{N}_{2}}O<NO<{{N}_{2}}{{O}_{3}}<{{N}_{2}}{{O}_{4}}<{{N}_{2}}{{O}_{5}}\].
The decreasing order of stability of oxides of group 15 follows as,
\[{{P}_{2}}{{O}_{5}}>A{{s}_{2}}{{O}_{5}}>S{{b}_{2}}{{O}_{5}}>B{{i}_{2}}{{O}_{5}}\]
The nature of oxides of group 15 elements is as follows,
\[{{N}_{2}}{{O}_{3}}\] and \[{{P}_{2}}{{O}_{3}}\] (acidic) ; \[A{{s}_{2}}{{O}_{3}}\] and \[S{{b}_{2}}{{O}_{3}}\](amphoteric); \[B{{i}_{2}}{{O}_{3}}\] (basic)
(4) Oxyacids : \[{{N}_{2}}\] and \[{{P}_{4}}\] of this group forms oxyacids which are discussed further. In this chapter.
Anamalous behaviour of Nitrogen
Nitrogen is known to differ form other members of the family because of the following facts,
(1) Its small size (2) Its high electronegativity (3) Its high ionisation energy (4) non-availability of d-orbital in the valence shell. (5) Its capacity to form pp-pp multiple bonds.
The main points of difference are,
(i) Nitrogen is a gas while other members are solids.
(ii) Nitrogen is diatomic while other elements like phosphorus and arsenic form tetra-atomic molecules \[({{P}_{4}},A{{s}_{4}})\].
(iii) Nitrogen form five oxides \[({{N}_{2}}O,NO,{{N}_{2}}{{O}_{3}},{{N}_{2}}{{O}_{4}}\] and \[{{N}_{2}}{{O}_{5}})\] while other members of the family form two oxides (tri and pentaoxides).
(iv) Hydrides of nitrogen show H-bonding while those of other elements do not.
(v) Nitrogen does not show pentacovalency because of absence of d-orbitals while all other elements show pentacovalency.
(vi) Nitrogen dos not form complexes because of absence of d-orbitals while other elements show complex formation e.g., \[{{[PC{{l}_{6}}]}^{-}},{{[AsC{{l}_{6}}]}^{-}}\] etc.
(vii) The hydride of nitrogen \[(N{{H}_{3}})\] is highly basic in nature while the hydrides of other elements are slightly basic.
(viii) Except for \[N{{F}_{3}}\], other halides of nitrogen e.g., \[NC{{l}_{3}},NB{{r}_{3}}\] and \[N{{I}_{3}}\] are unstable.
Nitrogen and its compounds
\[{{N}_{2}}\] was discovered by Daniel Rutherford. It is the first member of group 15 in the periodic table.
Occurrence : \[{{N}_{2}}\], occurs both in the free state as well as in the combined state. \[{{N}_{2}}\] occurs in atmosphere to the extent of 78% by volume in free state. \[{{N}_{2}}\] is present in many compounds such as potassium nitrate (nitre). Sodium nitrate (Chile salt peter) and many ammonium compounds. \[{{N}_{2}}\] is an important constituent of proteins in plants and animals in combined state.
Preparation : It is prepared by the following methods,
(1) Laboratory method : In the laboratory \[{{N}_{2}}\] is prepared by heating an aqueous solution containing an equivalent amounts of \[N{{H}_{4}}Cl\] and \[NaN{{O}_{2}}\].
\[N{{H}_{4}}Cl\,(aq.)+NaN{{O}_{2}}(aq.)\xrightarrow{\text{Heat}}{{N}_{2}}(g)+2{{H}_{2}}O(l)+NaCl\]
(2) Commercial preparation : Commercially \[{{N}_{2}}\] is prepared by the fractional distillation of liquid air.
Physical properties : \[{{N}_{2}}\] is a colourless, odourless and tasteless gas. It is a non-toxic gas. It’s vapour denstiy is 14. It has very low solubility in water.
Chemical properties
(1) \[{{N}_{2}}\] is neutral towards litmus. It is chemically unreactive at ordinary temp. It is neither combustible nor it supports combustion.
(2) The N – N bond in \[{{N}_{2}}\] molecule is a triple bond \[(N\equiv N)\] with a bond distance of 109.8 pm and bond dissociation energy of 946 kJ mol-1
(3) Combination with compounds : \[{{N}_{2}}\] combines with certain compounds on strong heating . eg
\[\underset{\text{Calsium carbide}}{\mathop{Ca{{C}_{2}}}}\,+{{N}_{2}}\xrightarrow{1300K}\underset{\text{Calsium cyanamide}}{\mathop{CaC{{N}_{2}}+C}}\,\]
\[\underset{\begin{smallmatrix} \text{Aluminium} \\ \,\,\,\,\,\,\text{oxide}\end{smallmatrix}}{\mathop{A{{l}_{2}}{{O}_{3}}}}\,+{{N}_{2}}+3C\xrightarrow{2100K}\underset{\text{Al}\text{. nitride}}{\mathop{2AlN}}\,+3\,CO\]
Both these compounds are hydrolysed on boiling with water to give ammonia.
\[CaC{{N}_{2}}+3{{H}_{2}}O\xrightarrow{{}}CaC{{O}_{3}}+2N{{H}_{3}}\]
\[AlN+3{{H}_{2}}O\xrightarrow{{}}Al\,{{(OH)}_{3}}+N{{H}_{3}}\]
Therefore, calcium cyanamide is used as a fertilizer under the name nitrolim \[(CaC{{N}_{2}}+C)\]
Uses of nitrogen : \[{{N}_{2}}\] is mainly used in the manufacture of compounds like \[N{{H}_{3}},HN{{O}_{3}},CaC{{N}_{2}}\] etc.
Compounds of nitrogen
(1) Hydrides of nitrogen – Ammonia
Preparation of ammonia : Ammonia is prepared in the laboratory by heating a mixture of \[N{{H}_{4}}Cl\] and slaked lime, \[Ca{{(OH)}_{2}}\]
\[2N{{H}_{4}}Cl+Ca{{(OH)}_{2}}\xrightarrow{\Delta }CaC{{l}_{2}}+2N{{H}_{3}}+2{{H}_{2}}O\]
Moist \[N{{H}_{3}}\] gas is dried over quick lime, \[CaO\]. However, it cannot be dried over conc. \[{{H}_{2}}S{{O}_{4}},{{P}_{2}}{{O}_{5}}\] because being basic it forms salts with them. Anhydrous \[CaC{{l}_{2}}\] also cannot be used because it forms a complex \[CaC{{l}_{2}}.8N{{H}_{3}}\] with it.
Manufacture : (i) Ammonia is manufacture by Haber’s process. A mixture of pure \[{{N}_{2}}\] and \[{{H}_{2}}\] (in the ratio 1 : 3 by volume) is compressed to 200 – 1000 atmospheres and passed over finely divided \[Fe\](as catalyst) and \[Mo\](as promoter) at \[750K\]
\[{{N}_{2}}+3{{H}_{2}}\] \[\]\[2N{{H}_{3}}+93.6\,KJ\,mo{{l}^{-1}}\]
Favourable conditions for maximum yield of \[N{{H}_{3}}\] are :
(a) excess of reactants (\[{{N}_{2}}\]and \[{{H}_{2}}\]) (b) high pressure (c) low temperature and (d) use of catalyst and a promoter.
(ii) By the hydrolysis of calcium cyanamide \[(CaC{{N}_{2}})\] with super-heated steam at \[450K\]. \[CaC{{N}_{2}}\] itself is obtained by heating \[Ca{{C}_{2}}\] and \[{{N}_{2}}\] at \[1270K\].
\[Ca{{C}_{2}}+{{N}_{2}}\xrightarrow{\Delta }\,CaC{{N}_{2}}+C\]
\[CaC{{N}_{2}}+3{{H}_{2}}O\xrightarrow{450K}CaC{{O}_{3}}+2N{{H}_{3}}\]
Properties of NH3 : It is a colourless gas with pungent smell, highly soluble in \[{{H}_{2}}O\] and basic in nature. It liquefies on cooling under pressure to give liquid ammonia (bp. 240K). On heating, it causes intense cooling and hence is used as a refrigerant in ice, factories and cold storages.
It burns in excess of air to give \[{{N}_{2}}\] and \[{{H}_{2}}O\] and is oxidised to \[NO\] when passed over heate \[Pt\] at \[1075K\].
\[4N{{H}_{3}}+3{{O}_{2}}\xrightarrow{{}}2{{N}_{2}}+6{{H}_{2}}O\]
\[4N{{H}_{3}}+5{{O}_{2}}\xrightarrow{Pt.\,1075\,K}4NO+6{{H}_{2}}O\](ostwald process)
It reduces heated \[CuO\] to \[Cu\] and \[C{{l}_{2}}\] to \[HCl\] (which combines with \[N{{H}_{3}}\] to give \[N{{H}_{4}}Cl\]).
\[2N{{H}_{3}}+3CuO\xrightarrow{Heat}3Cu+3{{H}_{2}}O+{{N}_{2}}\]
\[\underset{\text{Excess}}{\mathop{8N{{H}_{3}}}}\,+3C{{l}_{2}}\xrightarrow{{}}6N{{H}_{4}}Cl+{{N}_{2}}\]
With excess of \[C{{l}_{2}}\], it gives \[NC{{l}_{3}}\]. With \[B{{r}_{2}}\] it gives \[N{{H}_{4}}Br\] and \[{{N}_{2}}\] is set free.
\[N{{H}_{3}}+\underset{\text{Excess}}{\mathop{3C{{l}_{2}}}}\,\xrightarrow{{}}NC{{l}_{3}}+3HCl\]
\[8N{{H}_{3}}+3B{{r}_{2}}\xrightarrow{{}}6N{{H}_{4}}Br+{{N}_{2}}\]
With \[{{I}_{2}}\], it gives nitrogen triiodide ammonia (brown ppt) which is explosive in dry state and decomposes when struck
\[2N{{H}_{3}}+3{{I}_{2}}\xrightarrow{{}}N{{H}_{3}}.N{{I}_{3}}+3HI\]
\[8N{{H}_{3}}.N{{I}_{3}}\xrightarrow{{}}5{{N}_{2}}+9{{I}_{2}}+6N{{H}_{4}}I\]
It forms amides with active metals like \[Na,\,K\] etc.
\[2Na+2N{{H}_{3}}\xrightarrow{575\,K}2NaN{{H}_{2}}+{{H}_{2}}\]
It forms complexes with many substances, e.g.,
\[[Ca{{(N{{H}_{3}})}_{6}}]\] \[C{{l}_{2}}\], \[\,[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{2}},\,[Cu{{(N{{H}_{3}})}_{4}}]\,S{{O}_{4}}\],
\[[Ag{{(N{{H}_{3}})}_{2}}]Cl\], \[[Cd{{(N{{H}_{3}})}_{4}}C{{l}_{2}}\] etc.
Its aqueous solution is weakly basic due to the formation of \[O{{H}^{-}}\] ions, \[N{{H}_{3}}+{{H}_{2}}O\xrightarrow{{}}NH_{4}^{+}+O{{H}^{-}}\]
With sodium hypochlorite in presence of glue or gelatine, excess of ammonia gives hydrazine
\[2N{{H}_{3}}+NaOCl\xrightarrow{{}}N{{H}_{2}}.N{{H}_{2}}+NaCl+{{H}_{2}}O\]
It undergoes self ionization in liquid state and acts as a solvent. \[2N{{H}_{3}}\xrightarrow{{}}NH_{4}^{+}+NH_{2}^{-}\]
Many polar compounds are soluble in liquid ammonia.
With Nessler’s reagent (an alkaline solution of \[{{K}_{2}}Hg{{l}_{4}})\], ammonia and ammonium salts give a brown precipitate due to the formation of Millon’s base.
\[{{K}_{2}}Hg{{I}_{4}}2KI+Hg{{I}_{2}}\]
\[Hg{{I}_{2}}+2N{{H}_{3}}\xrightarrow{{}}I-Hg-N{{H}_{2}}+N{{H}_{4}}I\]
\[2N{{H}_{2}}-Hg-I+{{H}_{2}}O\xrightarrow{{}}N{{H}_{2}}-Hg-O-Hg-I+N{{H}_{4}}I\]
or \[2{{K}_{2}}Hg{{I}_{4}}+N{{H}_{3}}+3KOH\xrightarrow{{}}{{H}_{2}}N-Hg-O-Hg-I+7KI+2{{H}_{2}}O\]
It is used as a refrigerant and in the manufacture of fertilizers.
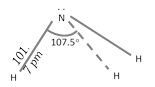
Strcture of NH3 : The \[N\] atom in \[N{{H}_{3}}\] is \[s{{p}^{3}}\]-hybridized containing a lone pair of electrons due to which the \[H-N-H\] bond angle is \[{{107.5}^{o}}\]. As a result \[N{{H}_{3}}\] molecule is pyramidal.
(2) Oxides of nitrogen : Nitrogen combines with \[{{O}_{2}}\] under different conditions to form a number of binary oxides which differ with respect to the oxidation state of the nitrogen atom. The important oxides are \[{{N}_{2}}O,NO,{{N}_{2}}{{O}_{3}},N{{O}_{2}},{{N}_{2}}{{O}_{4}}\] and \[{{N}_{2}}{{O}_{5}}\].
\[{{N}_{2}}O\] and NO both are neutral. Nitrous oxide (\[{{N}_{2}}O\]) has a sweet taste and its main use is as anaesthetic. When inhaled in mild quantities it causes hysterical laughter so it is also called Laughing gas. Nitric oxide (NO) can be obtained by treating a mixture of sodium nitrite and ferrous sulphate with dil. \[{{H}_{2}}S{{O}_{4}}\].\[{{N}_{2}}{{O}_{5}}\] is the strongest oxidising agent.
| Oxide | Structure | Physical appearance | Preparation |
| Nitrous oxide (\[{{N}_{2}}O\]) +1 | \[N\equiv N\to O\] | Colourless gas | By heating ammonium nitrate upto 2400C NH4NO3 \[\xrightarrow{\Delta }\] N2O + 2H2O, It is Collected over hot water |
| Nitric oxide (NO) +2 | N = O | Colourless | (a) By the action of cold dil. HNO3 on copper turnings (Laboratory method) 3Cu + 8 dil. HNO3 \[\to \]3Cu(NO3)2 + 4H2O + 2NO (b) By the action of H2SO4 on a mixture of FeSO4 and KNO3 (4:1) 2KNO3 + 5H2SO4 + 6FeSO4 \[\to \] 2KHSO4 + 3Fe2(SO4)3 + 4H2O + 2NO (c) By catalytic oxidation of ammonia. 4NH3 + 5O2 \[\underset{{{850}^{0}}C}{\overset{{}}{\mathop{\xrightarrow{Pt}}}}\,\]4NO + 6H2O |
| Dinitrogen trioxide (\[{{N}_{2}}{{O}_{3}}\]) +3 | Blue solid | (a) By the action of 50% HNO3 on arsenious oxide. 2HNO3 + As2O3 + 2H2O \[\to \]NO + NO2 + 2H3AsO4 \[\downarrow \] 250 K N2O3 | |
| Dinitrogen tetraoxide (\[{{N}_{2}}{{O}_{4}}\]) +4 | Colourless liquid | (a) By heating nitrates of heavy metals, e.g., lead nitrate. 2Pb(NO3)2 \[\xrightarrow{673\,K}\]4NO2 + 2PbO + 2O (b) By heating copper turnings with conc. HNO3. Cu + 4 HNO3 (conc.)\[\to \]Cu(NO3)2 + 2H2O + 2NO2 | |
| Nitrogen dioxide (\[N{{O}_{2}}\]) +4 | Brown gas | ||
| Dinitrogen pentoxide ( |
Colourless gas | (a) By dehydrating HNO3 with phosphorus pentoxide 4HNO3 +P4O10 |
(3) Oxyacids of nitrogen : Oxyacids of nitrogen are \[HN{{O}_{2}},HN{{O}_{3}},\] \[\underset{\text{(Nitroxylic acid)}}{\mathop{{{H}_{4}}{{N}_{2}}{{O}_{4}}}}\,\] and \[\underset{\text{(Pernitric acid)}}{\mathop{HN{{O}_{4}}}}\,\], which are explosive.
(i) Nitrous acid (HNO2) : It is prepared by adding ice cold dil, \[HCl\] or dil, \[{{H}_{2}}S{{O}_{4}}\] to a well cooled solution of any nitrite \[(NaN{{O}_{2}},\,Ba{{(N{{O}_{2}})}_{2}}\] etc.).
\[NaN{{O}_{2}}+HCl\xrightarrow{{}}NaCl+HN{{O}_{2}}\]
\[2KN{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}{{K}_{2}}S{{O}_{4}}+2HN{{O}_{2}}\]
It oxidises \[{{H}_{2}}S\] to \[S,\,Kl\] to \[{{I}_{2}}\] and acts as a reducing agent in presence of strong oxidising agent, i.e., it reduces acidified \[KMn{{O}_{4}},\,{{K}_{2}}C{{r}_{2}}{{O}_{7}},{{H}_{2}}{{O}_{2}}\] etc. to \[M{{n}^{2+}},\,C{{r}^{3+}}\] and \[{{H}_{2}}O\] respectively.
(ii) Nitric acid (HNO3) : \[HN{{O}_{3}}\] is called aqua fortis. It is prepared in the laboratory by distillation of nitre with conc. \[{{H}_{2}}S{{O}_{4}}\].
\[2NaN{{O}_{3}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}2HN{{O}_{3}}+N{{a}_{2}}S{{O}_{4}}\].
Commercially, it is obtained by Ostwald’s process. In this process, \[N{{H}_{3}}\] is first catalytically oxidised to \[NO\] which is cooled to about \[300K\] and then oxidised by air to \[N{{O}_{2}}\]. Absorption of \[N{{O}_{2}}\] in water in presence of oxygen gives \[HN{{O}_{3}}\]
\[4N{{H}_{3}}+5{{O}_{2}}\xrightarrow{Pt.\,1975\,K}4NO+6{{H}_{2}}O\]
\[2NO+{{O}_{2}}\rightleftharpoons 2N{{O}_{2}};\,4N{{O}_{2}}+2{{H}_{2}}O+{{O}_{2}}\xrightarrow{{}}4HN{{O}_{3}}\]
From air (Birkeland Eyde electric arc process)
\[{{N}_{2}}+{{O}_{2}}\rightleftharpoons 2NO;\,\Delta H_{f}^{o}=+135\,kJ\,mo{{l}^{-1}}\]
\[2NO+{{O}_{2}}\xrightarrow{{{50}^{o}}C}2N{{O}_{2}}\]
\[2N{{O}_{2}}+{{H}_{2}}O\xrightarrow{{}}HN{{O}_{2}}+HN{{O}_{3}}\]
\[3HN{{O}_{2}}\xrightarrow{{}}HN{{O}_{3}}+{{H}_{2}}O+2NO\]
Properties : It is a very strong acid and decomposes on boiling or in presence of sunlight. It acts as a strong oxidising agent. It oxidises nonmetals and metalloids to their respective oxy-acids, i.e., \[C\] to \[{{H}_{2}}C{{O}_{3}},\,S\] to \[{{H}_{2}}S{{O}_{4}},\,P\] to \[{{H}_{3}}P{{O}_{4}},\,{{l}_{2}}\] to \[Hl{{O}_{3}},\,As\] to \[{{H}_{3}}As{{O}_{4}}\](arsenic acid) and \[Sb\] to \[{{H}_{3}}Sb{{O}_{4}}\](antimonic acid), while nitric acid itself is reduced to \[N{{O}_{2}}\].
\[{{I}_{2}}+10HN{{O}_{3}}\xrightarrow{{}}2HI{{O}_{3}}+10N{{O}_{2}}+4{{H}_{2}}O\]
Nitric acid reacts with metals to form nitrates and is itself reduced to \[NO,\]\[{{N}_{2}}O,\,N{{O}_{2}}\] or \[N{{H}_{3}}\] (which further reacts with \[HN{{O}_{3}}\] to give \[N{{H}_{4}}N{{O}_{3}}\]) depending upon the concentration of the acid, activity of the metal and the temperature of the reaction.
(i) Very active metals such as \[Mn,\,Mg,\,Ca\], etc. give \[{{H}_{2}}\] on treatment with very dilute \[HN{{O}_{3}}\](2%).
(ii) Less active metals like \[Cu,\,Hg,\,Ag,\,Pb\] etc. give \[NO\] with dil. \[HN{{O}_{3}}\]. Zinc, however, gives \[{{N}_{2}}O\] with dil \[HN{{O}_{3}}\] and \[N{{H}_{4}}N{{O}_{3}}\] with very dilute \[HN{{O}_{3}}\].
\[Zn+10HN{{O}_{3}}\text{(dilute)}\xrightarrow{{}}4Zn{{(N{{O}_{3}})}_{2}}+{{N}_{2}}O+5{{H}_{2}}O\]
\[Zn+10HN{{O}_{3}}\text{(very dilute)}\xrightarrow{{}}4Zn{{(N{{O}_{3}})}_{2}}+N{{H}_{4}}N{{O}_{3}}+3{{H}_{2}}O\]
Similarly, \[Fe\] and\[Sn\] react with dilute nitric acid to give \[N{{H}_{4}}N{{O}_{3}}\].
(iii) Conc. \[HN{{O}_{3}}\] gives \[N{{O}_{2}}\] both with active metals \[(Zn,\,Pb\] etc.) and less active metals (\[Cu,\,Hg,\,Ag\] etc.)
\[Cu+4HN{{O}_{3}}\text{(Conc}\text{.)}\xrightarrow{{}}Cu{{(N{{O}_{3}})}_{2}}+2N{{O}_{2}}+2{{H}_{2}}O\]
Tin is, however, oxidized by conc. \[HN{{O}_{3}}\] to metastannic acid \[({{H}_{2}}Sn{{O}_{3}})\].
\[Sn+4HN{{O}_{3}}\xrightarrow{{}}{{H}_{2}}Sn{{O}_{3}}+4N{{O}_{2}}+{{H}_{2}}O\]
Passivity : \[Fe,Cr,\,Ni\] and \[Al\] become passive in conc. \[HN{{O}_{3}}\] (i.e., lose their normal reactivity) due to the formation of a thin protective layer of the oxide on the surface of the metal which prevents further action. Nitric acid has no action on noble metals \[(Au,\,Pt)\] but these metals dissolve in aqua regia (3 vol.\[HCl+\]1 vol.\[HN{{O}_{3}}\]) forming their respective chlorides.
\[HN{{O}_{3}}+3HCl\xrightarrow{{}}2{{H}_{2}}O+NOCl+2[Cl]\]
\[Au+3[Cl]\xrightarrow{{}}AuC{{l}_{3}};\]\[Pt+4[Cl]\xrightarrow{{}}PtC{{l}_{4}}\]
These chlorides subsequently dissolve in excess of \[HCl\] forming their corresponding soluble complexes. Thus,
\[\underset{\text{Auric chloride}}{\mathop{AuC{{l}_{3}}}}\,+HCl\xrightarrow{{}}\underset{\text{Aurochloric acid}}{\mathop{HAuC{{l}_{4}}}}\,\]
\[\underset{\text{Platinic chloride}}{\mathop{PtC{{l}_{4}}}}\,+2HCl\xrightarrow{{}}\underset{\text{Chloro platinic acid}}{\mathop{{{H}_{2}}PtC{{l}_{6}}}}\,\]
Sugar on oxidation with nitric acid gives oxalic acid. Nitric acid reacts with glycerine to give glycerol trinitrate or nitro glycerine, with toluene it gives 2, 4, 6-trinitrotoluene (T.N.T.) and with cellulose (cotton) it gives cellulose trinitrate (gun cotton). All these are used as explosives.
\[\underset{\text{Cane sugar}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+\underset{\text{From HN}{{\text{O}}_{\text{3}}}}{\mathop{18[O]}}\,\xrightarrow{{}}\,6\underset{\text{Oxalic acid}}{\mathop{\begin{matrix}COOH \\|\,\,\,\,\,\,\,\,\,\,\, \\ COOH \\\end{matrix}}}\,+5{{H}_{2}}O\]
Oxyacids of nitrogen
| Name of oxoacid | M. F. Formula M. F. | Structure | Oxidation State of N | Basicity | pKa | Nature |
| Hyponitrous acid | H2N2O2 | \[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,.\,\,.}{\mathop{\underset{HO-N}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,\,.\,\,.}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,N-OH}{\mathop{\,\,\,\,\,\,\,\,\,\,\,\,\,||}}\,}}\,}}\,}}\,\] | +1 | 2(dibasic) | Very weak | Highly explosive |
| Nitrous acid | HNO2 | \[H-\underset{\underset{{\mathrm O}}{\mathop{\downarrow }}\,}{\mathop{N}}\,=O\] | +3 | 1 (monobasic) | 3.3 | Unstable, Weak acid |
| Nitric acid | HNO3 | \[H-O-\underset{\underset{{\mathrm O}}{\mathop{\downarrow }}\,}{\mathop{N}}\,=O\] | +5 | 1 (monobasic) | -3.0 | Stable, Strong acid |
| Pernitric acid | HNO4 | O=\underset{\underset{{\mathrm O}}{\mathop{\downarrow }}\,}{\mathop{N}}\,-O-O-H\] | +5 | 1 (monobasic) | Unstable and explosive |
Phosphorus and its compounds
It is the second member of group 15 (VA) of the Periodic table. Due to larger size of P, it can not form stable \[P\pi -P\pi \] bonds with other phosphorous atoms where as nitrogen can form \[P\pi -P\pi \] bonds .
(1) Occurrence : Phosphorous occurs mainly in the form of phosphate minerals in the crust of earth. Some of these are:
(i) Phosphorite \[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}\], (ii) Fluorapatite \[C{{a}_{5}}{{(P{{O}_{4}})}_{3}}F\], (iii) Chlorapatite 3\[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}.CaC{{l}_{2}}\], (iv) Hydroxyapatite; \[C{{a}_{5}}{{(P{{O}_{4}})}_{3}}OH\]. Phosphates are essential constituents of plants and animals. It is mainly present in bones, which contains about 58% calcium phosphate.
(2) Isolation : Elemental phosphorus is isolated by heating the phosphorite rock with coke and sand in an electric furnace at about 1770K, \[2C{{a}_{3}}{{(P{{O}_{4}})}_{2}}+6Si{{O}_{2}}\xrightarrow{{}}\underset{\text{Calicum silicate }}{\mathop{6CaSi{{O}_{3}}}}\,+{{P}_{4}}{{O}_{10}}\]; \[{{P}_{4}}{{O}_{10}}+10C\xrightarrow{{}}{{P}_{4}}+10CO\]
(3) Allotropic forms of phosphorus : Phosphorus exists in three main allotropic forms,
(i) White phosphorus, (ii) Red phosphorus, (iii) Black phosphorus
(i) White or yellow phosphorus : It is obtained from phosphate rock or phosphorite as explained above. It exists as \[{{P}_{4}}\] units where four \[P\] atoms lie at the corners of a regular tetrahedron with \[\angle PPP={{60}^{o}}\]. Each \[P\] atom is linked to three other \[P\] atoms by covalent bonds. there are total six bonds and four lone pairs of electrons present in a \[{{P}_{4}}\] molecule of white phosphorus.
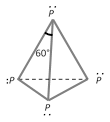
Properties : White phosphorus is extremely reactive due to strain in the \[{{P}_{4}}\] molecule, poisonous, soft, low melting \[(317K)\] solid, soluble in \[C{{S}_{2}}\], alcohols and ether. It has a garlic odour. Persons working with white \[P\] develop a disease known as Phossy jaw in which jaw bones decay. It turns yellow on exposure to light. Hence, it is also called yellow phosphorus.
It spontaneously catches fire in air with a greenish glow which is visible in the dark \[({{P}_{4}}+3{{O}_{2}}\to {{P}_{4}}{{O}_{6}})\]. This phenomenon is called phosphorescence. Because of its very low ignition temperature \[(303K)\], it is always kept under water.
With sulphur it gives tetraphoshorus trisulphide with explosive violence which is used in “strike anywhere matches”.
\[8{{P}_{4}}+3{{S}_{8}}\xrightarrow{{}}8{{P}_{4}}{{S}_{3}}\]
With metals phosphorus forms phosphides. For example,
\[{{P}_{4}}+6Mg\xrightarrow{{}}2M{{g}_{3}}{{P}_{2}}\]
With aqueous alkalies, on heating, white phosphorus gives phosphine
\[\overset{0}{\mathop{{{P}_{4}}}}\,+3NaOH+3{{H}_{2}}O\xrightarrow{{}}\]\[\underset{\text{(Phosphine)}}{\mathop{\overset{-3}{\mathop{P{{H}_{3}}}}\,}}\,+\underset{\text{Sod}\text{. hypophosphite}}{\mathop{3Na{{H}_{2}}\overset{+1}{\mathop{P{{O}_{2}}}}\,}}\,\]
It is an example of a disproportionation reaction where the oxidation state of \[P\] decreases from 0 to –3 (in \[P{{H}_{3}}\]) and increases to +1 (in \[Na{{H}_{2}}P{{O}_{2}}\])
White phosphorus acts as a strong reducing agent. It reduces \[HN{{O}_{3}}\] to \[N{{O}_{2}}\] and \[{{H}_{2}}S{{O}_{4}}\] to \[S{{O}_{2}}\]. It also reduces solutions of \[Cu,\,Ag\] and \[Au\] salts to their corresponding metals. For examples,
\[{{P}_{4}}+8CuS{{O}_{4}}+14{{H}_{2}}O\xrightarrow{{}}8Cu+8{{H}_{2}}S{{O}_{4}}+4{{H}_{3}}P{{O}_{4}}\]
\[{{P}_{4}}+20AgN{{O}_{3}}+16{{H}_{2}}O\xrightarrow{{}}20Ag+4{{H}_{3}}P{{O}_{4}}+20HN{{O}_{3}}\]
(ii) Red phosphorus : It is obtained by heating white phosphorus at \[540-570K\] out of contact with air in an inert atmosphere (\[C{{O}_{2}}\] or coal gas) for several hours.
White phosphorus \[\underset{C{{O}_{2}}\,\text{or coal gas}}{\mathop{\xrightarrow{540-570K}}}\,\] Red phosphorus
Red phosphorus exists as chains of \[{{P}_{4}}\] tetrahedra linked together through covalent bonds to give a polymeric structure as shown.
![]()
Due to its polymeric structure, red phosphorus is much less reactive and has m.p. much higher than that of white phosphorus.
Properties : Red phosphorus is a hard, odourless, non poisonous solid, insoluble in organic solvents such as \[C{{S}_{2}}\], alcohol and ether. Its ignition temperature is much higher than that of white phosphorus and thus does not catch fire easily. It does not show phosphorescence.
It sublimes on heating giving vapours which condense to give white phosphorus. It is denser than white phosphorus and is a bad conductor of electricity.
It burns in oxygen at \[565K\] to give phosphorus pentoxide, reacts with halogens, sulphur and alkali metals only when heated forming their corresponding salts.
It does not react with caustic alkalies and this property is made use in separating red phosphorus from white phosphorus.
(iii) Black phosphorus : It is obtained by heating white phosphorus at \[470K\] under high pressure (4000–12000atm) in an inert atmosphere.
White phosphorus \[\underset{4000-12000\,\text{atm}\text{. pressue}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\,\,\,\,\,\,470K\,\,\,\,\,\,\,\,\,\,\,}}}\,\]Black phosphorus
It has a double layered structure. Each layer is made up of zig-zag chains with \[P-P-P\]bond angle of \[{{99}^{o}}\]. Since it is highly polymeric, it has high density. It is the most stable (inactive) form of phosphorus and has a black metallic luster. It is a good conductor of heat and electricity.
(4) Compounds of phosphorus
(i) Oxides and oxyacids of phosphorus : Phosphorus is quite reactive and forms number of compounds in oxidation states of –3 , +3 and +5. Phosphorus forms two common oxides namely, (a) phosphorus trioxide (\[{{P}_{4}}{{O}_{6}}\]) and (b) phosphorus penta oxide \[({{P}_{4}}{{O}_{10}})\].
(a) Phosphours (III) oxide \[({{P}_{4}}{{O}_{6}})\]:
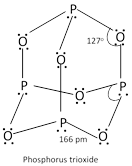
It is formed when P is burnt in a limited supply of air, \[{{P}_{4}}+\underset{\text{(limited)}}{\mathop{3{{O}_{2}}}}\,\]\[\to \]\[{{P}_{4}}{{O}_{6}}\].
It is a crystalline solid with garlic odour. It dissolves in cold water to give phosphorous acid,
\[{{P}_{4}}{{O}_{6}}\]+\[\underset{\text{cold}}{\mathop{6{{H}_{2}}O}}\,\]\[\to \]\[\underset{\text{Phosphorous acid}}{\mathop{4{{H}_{3}}P{{O}_{3}}}}\,\], It is therefore, considered as anhydride of phosphorus acid.
With hot water, it gives phosphoric acid and inflammable phosphine, \[{{P}_{4}}{{O}_{6}}+6{{H}_{2}}O\](hot)\[\to \] \[\underset{\text{Phosphoric acid}}{\mathop{3{{H}_{3}}P{{O}_{4}}+}}\,P{{H}_{3}}\]
It reacts vigorously with \[C{{l}_{2}}\]to form a mixture of phosphoryl chloride and meta phosphoryl chloride.
\[{{P}_{4}}{{O}_{6}}+4C{{l}_{2}}\to \underset{\text{Phosphoryl chloride}}{\mathop{2POC{{l}_{3}}}}\,+\underset{\text{Metaphosphoryl chloride}}{\mathop{2P{{O}_{2}}Cl}}\,\]
(b) Phosphorus (V) oxide \[\left( {{P}_{4}}{{O}_{10}} \right)\]:
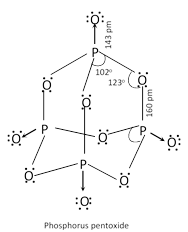
It is prepared by heating white phosphorus in excess of air, \[{{P}_{4}}+5{{O}_{2}}(excess)\to {{P}_{4}}{{O}_{10}}\]. It is snowy white solid. It readily dissolves in cold water forming metaphosphoric acid.
\[{{P}_{4}}{{O}_{10}}+\underset{\text{(Cold)}}{\mathop{2{{H}_{2}}O}}\,\to \underset{\text{Metaphosphoric acid}}{\mathop{4HP{{O}_{3}}}}\,\].
With hot water, it gives phosphoric acid, \[{{P}_{4}}{{O}_{10}}+\underset{\text{Hot}}{\mathop{6{{H}_{2}}O}}\,\to \underset{\text{Phosphoric acid}}{\mathop{4{{H}_{3}}P{{O}_{4}}}}\,\].
\[{{P}_{4}}{{O}_{10}}\] is a very strong dehydrating agent. It extracts water from many compounds including \[{{H}_{2}}S{{O}_{4}}\] and \[HN{{O}_{3}}\],
\[{{H}_{2}}S{{O}_{4}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{P}_{4}}{{O}_{10}}}}}\,S{{O}_{3}}\];\[2HN{{O}_{3}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{P}_{4}}{{O}_{10}}}}}\,{{N}_{2}}{{O}_{5}}\]
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{P}_{4}}{{O}_{10}}}}}\,\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}CN}}\,\]
(ii) Oxyacids of phosphorus : Phosphorus forms a number of oxyacids which differs in their structure and oxidation state of phosphorus. These are \[{{H}_{3}}P{{O}_{2}},\,{{H}_{3}}P{{O}_{3}},\,{{H}_{4}}{{P}_{2}}{{O}_{6}},\,{{H}_{3}}P{{O}_{4}},\,\] \[{{(HP{{O}_{3}})}_{n}},\,{{H}_{4}}{{P}_{2}}{{O}_{5}},\,{{H}_{4}}{{P}_{2}}{{O}_{7}}\]. From these \[{{H}_{3}}P{{O}_{2}},\,{{H}_{3}}P{{O}_{3}}\] are reducing agents. \[{{H}_{4}}{{P}_{2}}{{O}_{5}}\] (pyrophosphoric acid) is dibasic acid.
\[{{(HP{{O}_{3}})}_{n}}\] is formed by dehydration of \[{{H}_{3}}P{{O}_{4}}\] at \[{{316}^{o}}C\].
Oxyacids of phosphorus
| Name | Oxidation state of P and Basicity | Structure |
|
Hypophosphorous acid \[{{H}_{3}}P{{O}_{2}}\] |
+1 Monobasic | 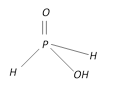 |
|
Phosphorous acid \[{{H}_{3}}P{{O}_{3}}\] |
+3 Dibasic |  |
|
Hypophosphoric acid \[{{H}_{4}}{{P}_{2}}{{O}_{6}}\] |
+4 Tetrabasic | 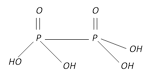 |
|
Orthophosphoric acid \[{{H}_{3}}P{{O}_{4}}\] |
+5 Tribasic | 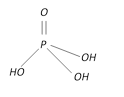 |
|
Metaphosphoric acid \[{{(HP{{O}_{3}})}_{n}}\] |
+5 Monobasic | 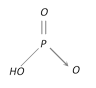 |
|
Pyrophospric acid (Diphosphoric acid). \[{{H}_{4}}{{P}_{2}}{{O}_{7}}\] |
+5 Tetrabasic | 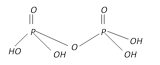 |
(5) Chemical Fertilizers : The chemical substances which are added to the soil to keep up the fertility of soil are called fertilizers.
Types of fertilizers : Chemical fertilizers are mainly of four types,
(i) Nitrogenous fertilizers : e.g. Ammonium sulphate \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}},\] Calcium cyanamide
\[CaC{{N}_{2}},\] Urea \[N{{H}_{2}}CON{{H}_{2}}\] etc.
(ii) Phosphatic fertilizers : e.g.\[Ca{{({{H}_{2}}P{{O}_{4}})}_{2}}.{{H}_{2}}O\,\](Triple super phosphate), Phosphatic slag etc.
(iii) Potash fertilizers : e.g. Potassium nitrate \[(KN{{O}_{3}}),\] Potassium sulphate \[({{K}_{2}}S{{O}_{4}})\]etc.
(iv) Mixed fertilizers : These are made by mixing two or more fertilizers in suitable proportion. e.g. NPK (contains nitrogen, phosphorus and potassium).
NPK is formed by mixing ammonium phosphate, super phosphate and some potassium salts.
You need to login to perform this action.
You will be redirected in
3 sec
