Carbon Family
Category : JEE Main & Advanced
Carbon is the first member of group 14 or IVA of the periodic table. It consists of five elements carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb). Carbon and silicon are nonmetals, germanium is metalloid and tin and lead are metals.
(1) Electronic configuration
| Elements | Electronic configuration (\[n{{s}^{2}}\ n{{p}^{2}}\]) |
| \[_{6}C\] | \[[He]\,2{{s}^{2}}2{{p}^{2}}\] |
| \[_{14}Si\] | \[[Ne]\,3{{s}^{2}}3{{p}^{2}}\] |
| \[_{32}Ge\] | \[[Ar]\,3{{d}^{10}}4{{s}^{2}}4{{p}^{2}}\] |
| \[_{50}Sn\] | \[[Kr]\,4{{d}^{10}}5{{s}^{2}}5{{p}^{2}}\] |
| \[_{82}Pb\] | \[[Xe]\,4{{f}^{14}}5{{d}^{10}}6{{s}^{2}}6{{p}^{2}}\] |
Physical Properties
(1) Non-metallic nature : The non-metallic nature decreases along the group.
C Si Ge Sn Pb
Non-metals metalloid metal metal \[\]
(2) Abundance : Carbon and silicon are most abundant elements in earth’s crust whereas germanium occurs only as traces. Tin and lead also occur in small amounts. Only carbon occurs in free state as coal, diamond and graphite and in combined state as carbonates, CO2 petroleum and natural gas Silicon is the second most abundant element after oxygen in earth’s crust in form of silicates and silica. Germanium found in traces in coal and in certain deposits. It important constituent for making conductors and transistors The important ore of tin is tin stone (SnO2) or cassiterite. Lead is found is form of galena (PbS) anglesite (PbSO4) and cerussite (PbCO3) The abundance ratio in earth’s crust is given below,
(3) Density : The density of these elements increases down the group as reported below
Element C Si Ge Sn Pb
Density (g/ml) 3.51 (for diamond) 2.34 5.32 7.26 11.34
2.22 (for graphite)
(4) Melting point and boiling points
(i) The melting point and boiling point of this group members decrease down the group.
Element C Si Ge Sn Pb
m.pt(K) 4373 1693 1218 505 600
b.pt.(K) – 3550 3123 2896 2024
(ii) The melting point and boiling point of group 14 elements are however, higher than their corresponding group 13 elements. This is due to the formation of four covalent bonds on account of four electrons in their valence shells which results in strong binding forces in between their atoms in solid as well as in liquid state.
(5) Atomic radii and atomic volume
(i) Both atomic radii and atomic volume increases gradually on moving down the group due to the effect of extra shell being added from member to member.
C Si Ge Sn Pb
Atomic radius (pm) 0.77 111 122 141 144
Atomic volume (ml) 3.4 11.4 13.6 16.3 18.27
(ii) The atomic radii of group 14 elements are than their corresponding group 13 elements due to increase in nuclear charge in the same period.
(iii) Some of the ionic radii involving six co-ordination of these group elements are given below,
C Si Ge Sn Pb
Ionic radius (M2+) in pm – – 73 118 119
Ionic radius (M++) in pm – 40 53 69 78
(6) Electronegativity : The electronegativity decreases from C to Si and then becomes constant.
C Si Ge Sn Pb
Electronegativity on pauling scale 2.5 1.8 1.8 1.7 1.6
The electronegativity from silicon onwards is almost is almost constant or shows a comparatively smaller decreases due to screening effects of d10 electrons in elements from Ge onwards.
(7) Ionisation energy
(i) The ionisation energy decreases regularly down the group; Pb however shows a higher value than Sn due to poor shielding of inner f-orbitals as a result of which effective nuclear charge experienced by outer shell electrons becomes more in Pb.
Ionisation energy (kJ mol-1) C Si Ge Sn Pb
IE1 1086 786 761 708 715
IE2 2352 1577 1537 1411 1450
(ii) The first ionisation energies of group 14 elements are higher than their corresponding group 13 elements because of smaller size.
(iii) The electropositive character of these elements increases down the group because of decreases in ionisation energy.
(8) Oxidation state
(i) Presence of four electrons in outermost shell of these elements reveals that the members of this family can gain four electrons forming M4+ or M4- ions to show ionic nature or exhibit tetravalent covalent nature by sharing of four electron pairs in order to attain stable configuration.
(ii) The formation of M4+ or M4- ions require huge amount of energy which is normally not available during normal course of reactions, therefore, these elements usually do not form M4+ or M4- ions, but they usually form compounds with covalence of four.
(iii) Ge, Sn and Pb also exhibit +2 oxidation state due to inert pair effect.
(iv) Sn2+ and Pb2+ show ionic nature.
(v) The tendency to form +2 ionic state increases on moving down the group due to inert pair effect.
(9) Catenation
(i) The tendency of formation of long open or closed atom chains by the combination of same atoms in themselves is known as catenation.
(ii) The catenation is maximum in carbon and decreases down the group.
(iii) This is due to high bond energy of catenation.
(iv) Only carbon atoms also form double or triple bonds involving pp-pp multiple bond within itself.
> C = C<; – C º C –
(v) Carbon also possesses the tendency to form closed chain compounds with O, S and N atoms as well as forming pp-pp multiple bonds with other elements particularly nitrogen and oxygen e.g. C =O; C=N; C º N; C = S are the functional groups present in numerous molecules due to this reason.
(vi) Carbon can form chain containing any number of carbon atoms Si and Ge cannot extend the chain beyond 6 atoms, while Sn and Pb do not form chains containing more than one or two atoms.
(vii) The reason for greater tendency of carbon for catenation than other elements in the group may further be explained by the fact that the C – C bond energy is approximately of the same magnitude as the energies of the bond between C and other elements. On the other hand, the Si – Si bond is weaker than the bond between silicon and other elements.
|
Bond |
Bond energy (k J/mol) |
Bond |
Bond energy (kJ/mol) |
|
C–C C–O C–H C–Cl C–F |
348 315 414 326 439 |
Si–Si Si–O Si–H Si–Cl Si–F |
180 372 339 360 536 |
(10) Allotropy
The phenomenon of existence of a chemical element in two or more forms differing in physical properties but having almost same chemical nature is known as allotropy. If an element or compound exists in two or more forms, it is also known as polymorphism e.g. zinc blende and wurtzite are polymorphs of ZnS.
Kinds of allotropy. Allotropy is of three types :
(i) Enantiotropy : When two forms of a solid substance exist together in equilibrium with each other at a particular temperature under normal pressure it is called enantiotropy.
For example, at normal pressure and temperature between \[368.6\,K\] and \[285K\], sulphur (solid) exist in two forms (rhombic sulphur), \[{{S}_{R}}\] and (monoclinic sulphur), \[{{S}_{M}}\] in equilibrium with each other. \[{{S}_{R}}\]\[\rightleftharpoons \]\[{{S}_{M}}\]
(ii) Monotropy : It is the type of allotropy in which only one allotrope is stable, under normal conditions the other being unstable e.g., diamond and graphite, oxygen and ozone etc.
(iii) Dynamictropy : It is the type of allotropy in which there is a true equilibrium between the two allotropes, one changing into the other at exactly the same rate as the reverse occurs. Both allotropes are stable over a wide range of temperature.
For example, liquid sulphur exist in two forms, the pale yellow mobile form called \[{{S}_{\lambda }}\] and dark viscous form called \[{{S}_{\mu }}\] in equilibrium with each other. \[{{S}_{\lambda }}\]\[\rightleftharpoons \]\[{{S}_{\mu }}\]
With increase in temperature, the later form is formed at the expense of the former but when the liquid is cooled, the reverse change occur. Thus sulphur shows both enantiotropy and dynamictropy.
Cause of allotropy : (i) In general the allotropy among solid substances is due to the difference in crystalline structure. (ii) It may also be due to the presence of different number of atoms e.g., \[{{O}_{2}}\] and \[{{O}_{3}},\,{{S}_{8}},\,{{S}_{2}}\] etc. (iii) It may be due to the difference in nuclear spins e.g., ortho and para hydrogen.
Different allotropic forms Except lead, all elements show allotropy.
(i) Carbon has two crystalline allotropic forms i.e., diamond and graphite. In diamond \[C\] atom is \[s{{p}^{3}}\] hybridised and it has a three dimensional network structure. Since no valence electron is available, hence diamond is a bad conductor of electricity. However in graphite \[C\] atom is \[s{{p}^{2}}\] hybridised and has a delocalised \[\pi \]-electron cloud responsible for its high electrical conductivity. It may be noted that diamond is thermodynamically less stable than graphite at ordinary temperatures.
(ii) Silicon has both crystalline and amorphous forms.
(iii) Tin has three crystalline modifications with the following equilibrium temperature
\[\underset{\text{(Grey)}}{\mathop{\alpha -tin}}\,\underset{\text{(White)}}{\mathop{\beta -Sn}}\,\underset{\text{(Rhombic)}}{\mathop{\gamma -Sn}}\,Liquid\,tin\]
The conversion of white tin to grey tin is accompanied by an increase in volume and the latter, being very brittle, easily crumbles down to powder. This phenomenon is called tin disease tin pest or tin plague.
Chemical properties
(1) Hydrides : All the elements of group 14 combine with hydrogen directly or indirectly to form the covalent hydrides, \[M{{H}_{4}}\] (M = C, Si, Ge, Sn or Pb). The number of hydrides and the ease of preparation decrease on going from carbon to lead.
The hydrides of silicon are called silanes having the general formula \[S{{i}_{n}}{{H}_{2n+2}}\]. The hydrides of germanium are called germanes while those of tin are called the stannanes. Only lead forms an unstable hydride of the formula, \[Pb{{H}_{4}}\] called the plumbane.
Three hydrides of germanium, i.e., \[Ge{{H}_{4}},G{{e}_{2}}{{H}_{6}}\] and \[G{{e}_{3}}{{H}_{8}}\] and only two hydrides of tin i.e., \[Sn{{H}_{4}}\] and \[S{{n}_{2}}{{H}_{6}}\] are well known.
(2) Oxides : Carbon forms five oxides \[CO,C{{O}_{2}},{{C}_{3}}{{O}_{2}}\] (carbon suboxide), \[{{C}_{5}}{{O}_{2}}\] and \[{{C}_{12}}{{O}_{9}},{{C}_{3}}{{O}_{2}}\] is the anhydride of malonic acid and \[C{{O}_{2}}\] is the anhydride of \[{{H}_{2}}C{{O}_{3}}\] (carbonic acid) \[C{{O}_{2}}\] is a non-polar linear molecule due to maximum tendency of C to form pp–pp multiple bond with oxygen. Si forms \[Si{{O}_{2}}\]. Pb forms a number of oxides. PbO can be obtained by heating \[Pb{{(N{{O}_{3}})}_{2}}\], \[2Pb{{(N{{O}_{3}})}_{2}}\xrightarrow{\text{Heat}}2PbO+4N{{O}_{2}}+{{O}_{2}}\].
The red form of PbO is called litharge and the yellow form is massicot. \[P{{b}_{3}}{{O}_{4}}\](Red lead, or Sindur) is prepared by heating litharge in air at 470°C, \[6PbO+{{O}_{2}}\xrightarrow{{{470}^{o}}C}2P{{b}_{3}}{{O}_{4}}\], \[P{{b}_{3}}{{O}_{4}}\] is a mixed oxide of \[Pb{{O}_{2}}.2PbO.\,P{{b}_{2}}{{O}_{3}}\] is called lead sesquioxide. \[Ge{{O}_{2}},Sn{{O}_{2}}\] etc. are also network solids.
\[C{{O}_{2}}\] and \[Si{{O}_{2}}\] is acidic, \[Ge{{O}_{2}}\] is weakly acidic while \[Sn{{O}_{2}}\] and \[Pb{{O}_{2}}\] are amphoteric in nature.
All the elements of group 14 except silicon from monoxides e.g., \[CO,GeO,SnO\] and PbO. Out of these monoxides only CO is neutral, while all other monoxides are basic.
(3) Halides : Elements of group 14 react with halogens directly to form tetrahedral and covalent halides except C where its halide is produced by the action of halogens on hydrocarbons. \[PbB{{r}_{4}}\] and \[Pb{{I}_{4}}\] do not exist because \[P{{b}^{4+}}\] is a strong oxidant and \[B{{r}^{-}}\] and \[{{I}^{-}}\] are strong reductants. Hence \[P{{b}^{4+}}\] ion is difficult to survive in presence of strong reductants \[B{{r}^{-}}\] and \[{{I}^{-}}\] and is immediately reduced to \[P{{b}^{2+}}\].
(4) Carbides : Carbides are binary compounds of carbon with elements of lower or about equal electronegativity.
Preparation : Carbides are generally prepared by heating the elements orits oxide with carbon or hydrocarbon at very high temperatures.
\[Ca+2C\xrightarrow{{}}Ba{{C}_{2}}\]; \[2Li+2C\xrightarrow{{}}L{{i}_{2}}{{C}_{2}}\]
\[CaO+3C\xrightarrow{{}}Ca{{C}_{2}}+CO\]
\[4Li+{{C}_{2}}{{H}_{2}}\xrightarrow{{}}L{{i}_{2}}{{C}_{2}}+LiH\]
Carbides are classified into three types on the basis of chemical bonding.
(1) Salt like carbides : These carbides are formed by the metals of groups IA, IIA, IIIA (except boron), coinage metals, Zinc, cadmium & some lanthanides.
(i) Acetylides : These are ionic carbides which yield acetylene on hydrolysis. The alkali metals and copper, silver and gold form M2C2 type compounds. These contain \[C_{2}^{2-}\] ions.
(ii) Methanides : These carbides evolve methane on hydrolysis. \[A{{l}_{4}}{{C}_{3}},\,B{{e}_{2}}C,\,M{{n}_{3}}C\] etc are some are of methanides. These contains \[{{C}^{4-}}\] groups.
(iii) Allylides : These carbides evolve allylene (methyl acetylene) on hydrolysis. This type of the carbides is only \[M{{g}_{2}}{{C}_{3}}\] it contains \[C_{3}^{4-}\] discrete groups.
(2) Mixed carbides : These carbides yield a mixture of hydrocarbons on hydrolysis, carbides of iron group, \[U{{C}_{2}}\] and \[Th{{C}_{2}}\] belong to this group.
(3) Covalent carbides : The only true covalent carbides are those of \[SiC\] (carborundum) and \[{{B}_{4}}C,\,{{B}_{13}}{{C}_{2}}\] etc. These are chemicallyinert so become hard.
On account of hardness, these are used as abrasives.
(4) Metallic or interstitial carbides : If these carbides possess metallic lustre high electrical conductivity and chemically inert. These are extremely hard like diamond and possess very high melting points.
Ability to form complexes : The ability of group 14 elements to form complexes is highly favoured by a high charge, small size and availability of empty orbitals of the right energy. The compounds in which carbon shows a covalency of four possess a closed shell electronic configuration of a noble gas and therefore carbon does not form complexes. Silicon and other heavier elements, however, can form complexes due to the availability of energetically suitable empty \[d\]-orbitals and a coordination number of six is found in these complexes. For example, in the formation of \[{{[Si{{F}_{6}}]}^{2-}}\], four covalent and two co-ordinate bonds are formed as a result of \[s{{p}^{3}}{{d}^{2}}\] hybridisation. As such the resulting ion has an octahedral geometry. Thus elements like \[Si,\,Ge,\,Sn\] and \[Pb\] have an ability to increase their co-ordination number from four to six. Other examples of hexa co-ordianted species are :
\[{{[Ge{{F}_{6}}]}^{2-}},\,{{[SnC{{l}_{6}}]}^{2-}},\,{{[PbC{{l}_{6}}]}^{2-}}\] etc.
Anomalous behaviour of Carbon
Carbon is found differ in many properties from the rest of the members of group 14. This is because of the following : (i) Its smallest size (ii) Its high electronegativity (iii) Its property to catenate (iv) Absence of d-orbitals in it.
Some of the properties in which it differs from other members are,
(1) The melting and boiling points of carbon are very high as compared to the rest to the members of the family.
(2) Carbon in its diamond form is one of the hardest substance known.
(3) It has maximum tendency to show catenation.
(4) Carbon has high tendency to form Pp – Pp multiple bonds with other elements like nitrogen, oxygen, sulphur etc. Other members of the family form Pp – dp bonds and that also to a lesser extent.
(5) \[C{{O}_{2}}\] is a gas while the dioxides of all other members are solids.
(6) Carbon is not affected by alkalies whereas other members react on fusion. For example, silicon form silicates, \[Si+2NaOH+1/2\,{{O}_{2}}\to \underset{\text{Sodium silicate }}{\mathop{N{{a}_{2}}Si{{O}_{3}}}}\,+{{H}_{2}}\].
Silicon and its compounds
Silicon, being a second member of group – 14, has a much larger size and lower electronegativity than that of carbon. As a result silicon does not form double bond with itself or with oxygen. Thus SiO bonds are much stronger than Si – Si and Si –H bonds. Silicon has vacant 3d-orbitals in its valence shell due to which it can extend its covalency from four to five and six.
(1) Occurrence : Silicon is the second most abundant element ( 27.7%) in earth’s crust next to oxygen .It does not occur in free state. It occurs mainly in the form of Silica and silicates. Silicates are formed in rocks and clay as silicates of Mg, Al, K or Fe. e.g. Feldspar ; \[{{K}_{2}}A{{l}_{2}}{{O}_{3}}.6Si{{O}_{2}}\], Kaolinite; \[A{{l}_{2}}{{O}_{3}}.2Si{{O}_{2}}.2{{H}_{2}}O\].
(2) Preparation : Elemental silicon is obtained by reduction of silica with high purity coke in an electric furnace using excess of silica e.g. \[Si{{O}_{2}}+2C\xrightarrow[{}]{}Si+2CO\]
Very high purity silicon required for making semiconductors is obtained by reduction of highly purified \[SiC{{l}_{4}}\] form (\[SiHC{{l}_{3}}\]) with hydrogen followed by purification by zone refining eg.
\[SiC{{l}_{4}}+2{{H}_{2}}\xrightarrow{{}}Si+4HCl\]; \[SiHC{{l}_{3}}+{{H}_{2}}\xrightarrow{{}}Si+3HCl\]
(3) Properties : (i) Silicon exists in three isotopes \[_{14}^{{}}S{{i}^{29}}\] (most common), \[_{14}^{{}}S{{i}^{30}}\]with air at high temperature \[Si{{O}_{2}}\] form,
Si + \[{{O}_{2}}\]\[\xrightarrow{{}}\] Si\[{{O}_{2}}\].
(ii) With steam, Si reacts when heated to redness to liberate hydrogen, Si + 2\[{{H}_{2}}O\] \[\xrightarrow{\text{Redness}}\]Si\[{{O}_{2}}\]+ 2\[{{H}_{2}}\].
(iii) With halogens, Si reacts at elevated temperature forming \[Si{{X}_{4}}\] except fluorine which reacts at room temperature.
(iv) Silicon combines with C at 2500K forming Silicon Carbide (SiC) known as carborundum (an extremely hard substance),
\[Si+C\xrightarrow{\text{2500 K}}SiC\].
(v) It reacts with metals like Ca, Mg etc in an electric arc furnace to form Silicides (\[C{{a}_{2}}Si,M{{g}_{2}}Si\]etc.)
(vi) Silicon dissolves in hot aqueous alkalies liberating hydrogen, Si + 4NaOH \[\xrightarrow{Heat}\]\[N{{a}_{4}}Si{{O}_{4}}+2{{H}_{2}}\uparrow \]
(vii) It also dissolves in fused \[N{{a}_{2}}C{{O}_{3}}\] displacing carbon \[N{{a}_{2}}SiO{}_{3}+C\].
(4) Uses of silicon : (i) It is added to steel as ferrosilicon ( an alloy of Fe and Si) to make it acid resistant.
(ii) It is used in the pure form as a starting material for production of silicon polymers (Silicones).
(5) Compounds of silicon
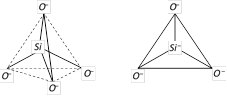
(i) Silicates : Silicates are the metal derivatives of silicic acid (H4SiO4). The basic of all silicates is the \[SiO_{4}^{4-}\]anion. In \[SiO_{4}^{4-}\] anion, Si is sp3 hybridised, and it forms four covalent bonds with four negatively charged oxygen atoms. \[SiO_{4}^{4-}\] anion has a tetrahedral shape.
Alkali metal silicates are commonly prepared by fusing metal oxides or metal carbonates with sand (SiO2) at high temperatures. For examples, sodium silicate can be prepared by fusing sand with sodium carbonate.
\[N{{a}_{2}}C{{O}_{3}}+\underset{\text{sand}}{\mathop{Si{{O}_{2}}}}\,\xrightarrow{\text{Fusion}}\underset{\text{sodium silicate}}{\mathop{N{{a}_{2}}Si{{O}_{3}}}}\,+C{{O}_{2}}(g)\] Classification of silicates
| No. of carners of SiO4 tetrahe-dra shared | No. of com-mon oxygen atoms | Net cha-rge and Anion in the silicate structure | Example | |
| Diagram and Description | ||||
| Zero | Zero |
Discrete SiO4 4-anion |
\[\begin{align} & \frac{\begin{align} & Si=\text{ }+4 \\ & O=~\text{ }8 \\ \end{align}}{Net=4} \\ & SiO_{4}^{4-} \\ \end{align}\] | ortho-silicates \[M{{g}_{2}}Si{{O}_{4}}\] |
| 1 | 1 |
Island structure |
\[\begin{align} & \frac{\begin{align} & Si=\text{ }+8 \\ & O=~\text{ 14} \\ \end{align}}{Net=6} \\ & {{(S{{i}_{2}}{{O}_{7}})}^{6-}} \\ \end{align}\] | Pyro- silicates |
| 2 | 2 |
Ring anion |
\[\begin{align} & \frac{\begin{align} & Si=\text{ }+12 \\ & O=~\text{ 18} \\ \end{align}}{Net=6} \\ & {{(S{{i}_{3}}{{O}_{9}})}^{6-}} \\ \end{align}\] | Wollas-tonite Ca3Si3O9 |
| 2 | 2 | Ring anion | \[\begin{align} & \frac{\begin{align} & Si=\text{ }+24 \\ & O=~\text{ 36} \\ \end{align}}{Net=12} \\ & {{(S{{i}_{6}}{{O}_{18}})}^{12-}} \\ \end{align}\] | Beryl, \[B{{e}_{3}}A{{l}_{2}}\] \[S{{i}_{6}}{{O}_{18}}\] |
| 2 | 2 |
Chain anion |
\[\begin{align} & \frac{\begin{align} & Si=\text{ }+4 \\ & O=~\text{ 6} \\ \end{align}}{Net=2} \\ & {{(SiO_{3}^{2-})}_{n}} \\ \end{align}\] | Pyroxenes, e.g., \[MgCa\] \[S{{i}_{2}}{{O}_{6}}\] Asbestos |
| 3 | 3 |
Two dimensional sheet structure |
\[\begin{align} & \frac{\begin{align} & Si=\text{ }+8 \\ & O=~\text{ 10} \\ \end{align}}{Net=2} \\ & {{(S{{i}_{2}}O_{5}^{2-})}_{n}} \\ \end{align}\] | Clays, talc kaolinite |
| 4 | 4 | - Three dimensional network | \[\begin{align} & Si=\text{ }+8 \\ & O=~\text{ 10} \\ & {{(Si{{O}_{3}})}_{n}} \\ \end{align}\] | Quartz, Tridymite and Cristo-balite |
(ii) Silica or silicon dioxide (\[Si{{O}_{2}}\])
It occurs in nature in various forms such as sand, quartz and flint .It is also a constituent of various rocks. It is solid at room temperature. It is insoluble in water.
Silica has a three dimensional network structure in which each Si is bonded to four oxygen atoms which are tetrahedrally disposed around silicon atom. Each O atom is shared by two Si atoms. It may be noted that \[C{{O}_{2}}\]is a gas, while \[Si{{O}_{2}}\]is hard solid with very high melting point.
Si\[{{O}_{2}}\] + 4HF \[\xrightarrow{{}}\] Si\[{{F}_{4}}\]+ \[2{{H}_{2}}O\]
Si\[{{F}_{4}}\]+ 2HF \[\xrightarrow{{}}\]\[\underset{\text{(Hydro flouro silicic acid)}}{\mathop{{{H}_{2}}Si{{F}_{6}}}}\,\]
HF readily dissolves Silica, therefore HF can not be store in glass bottles which contain Silica.
It is used in large amount to form mortar which is a building material. It is also used in the manufacture of glass and lenses.
(iii) Silicones
Polymeric organo-silicon compounds containing \[Si-O-Si\] bonds are called silicones. These have the general formula \[{{({{R}_{2}}SiO)}_{n}}\]. Where \[R\] is \[C{{H}_{3}}\]-group (majority cases) or \[{{C}_{6}}{{H}_{5}}\]-group.
Preparation : The preparation of silicones is generally carried out by the hydrolysis of dialkyldichlorosilanes \[({{R}_{2}}SiC{{l}_{2}})\] or diaryldichlorosilanes \[(A{{r}_{2}}SiC{{l}_{2}})\], which are prepared by passing vapours of \[RCl\] or \[ArCl\] over silicon at \[570K\] with copper as a catalyst.
\[2RCl+Si\xrightarrow{Cu,\,570K}{{R}_{2}}SiC{{l}_{2}}\]
\[{{R}_{2}}SiC{{l}_{2}}\underset{-HCl}{\mathop{\xrightarrow{+{{H}_{2}}O}}}\,\]\[-\begin{matrix}R\,\,\,\,\,\,\,\,\,\,\,\,\,R\,\,\,\,\,\,\,\,\,\,\,\,\,\,R\,\,\,\, \\|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\, \\O-Si-O-Si-O-Si-O- \\|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\, \\R\,\,\,\,\,\,\,\,\,\,\,\,\,\,R\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,R\, \\\end{matrix}\,\]
Silicones may be obtained in the form of oils, rubber on resins depending upon the extent of polymerisation which depends upon reaction conditions and nature of alkyl groups.
Properties and Uses : Silicones are water repellent and quite inert chemically. These resist oxidation, thermal decomposition and attack by organic reagents. These are also good electrical insulators and antifoaming agents. These have found the following uses :
(a) Silicones have been used for making water-proof papers, wools, textiles, wood etc., after coating these articles with silicones.
(b) The viscosities of silicones do not change with changes in temperature, therefore, these are used as all weather lubricants.
(c) As antifoaming agent in industrial processes.
(d) As a mould releasing agent in rubber industry and foundry. It avoids the sticking of the castings to the mould.
(e) For making body implants in cosmetic surgery due to its inert nature.
(f) Silicones are now incorporated in paints for resisting dampness and for water proofing.
(g) Due to their water repellent nature and high dielectric constant, silicones are used in electrical condensers.
(iv) Silica gel : When a mineral acid (Such as HCl) is added to a concentrated solution of a silicate, gelatinous white ppt. of hydrated silica (silicic acid) separate out.
\[N{{a}_{2}}Si{{O}_{3}}+2HCl\xrightarrow{{}}2NaCl+Si{{O}_{2}}.x{{H}_{2}}O\]
The white ppt. thus obtained is heated to lose water. When the water content is very low, the solid product is called silica gel. It possesses excellent absorptive properties due to its porous nature and is used for absorbing moisture and an adsorbent in chromatography.
(v) Silanes : The hydrides of silicon are called silanes. For example; \[Si{{H}_{4}}\] Silane, \[S{{i}_{2}}{{H}_{6}}\] disilane, \[S{{i}_{3}}{{H}_{8}}\] Trisilane \[S{{i}_{4}}{{H}_{10}}\] Tetrasilane.
Silanes are poisonous. These are much less stable than the corresponding alkanes and are decomposed into elements on heating above \[{{450}^{o}}C\]. Their thermal stability decreases with increase in molecular mass. Unlike alkanes, silanes are reducing agents.
(vi) Glass
Glass is an amorphous and transparent solid which is obtained by solidification of various silicates and borates of potassium and calcium.
Preparation : Ordinary glass is a mixture of sodium and calcium silicates and is produced by fusing together a mixture of sodium carbonate, calcium oxide and silicon dioxide (Silica) in a furnace at about 1700K
\[N{{a}_{2}}C{{O}_{3}}+Si{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}Si{{O}_{3}}+C{{O}_{2}}\uparrow \]
\[CaO+Si{{O}_{2}}\xrightarrow{{}}CaSi{{O}_{3}}\]
On continuously heating the entire amount of \[C{{O}_{2}}\]is driven out and clear viscous fused mass is obtained. It is poured into moulds to get different types of articles, which are allowed to cool gradually.
This typed of glass is called soda glass or soft glass which has the approximate composition, \[N{{a}_{2}}Si{{O}_{3}},CaSi{{O}_{3}},4Si{{O}_{2}}\].
Various varieties of glass : The different varieties of glasses and their special constituents are given below,
| Type of glass | Constituents | Special use |
| Soft glass | \[N{{a}_{2}}C{{O}_{3}},\,CaC{{O}_{3}},\]\[Si{{O}_{2}}\] | Ordinary glass for window panes, test tubes, bottles, etc. |
| Hard glass | \[{{K}_{2}}C{{O}_{3}},CaC{{O}_{3}},SiO{}_{2}\] | For combustion tubes and chemical glassware |
| High refractive index glass (Flint glass) | Lead oxide, \[{{K}_{2}}C{{O}_{3}}\] | For making lenses cut glasses |
| Pyrex glass | \[Na{}_{2}C{{O}_{3}},A{{l}_{2}}{{O}_{3}}\], \[{{B}_{2}}{{O}_{3}}\]or borax, sand | For high quality glass apparatus cooking utensils |
| Crook's glass | \[{{K}_{2}}C{{O}_{2}},\,PbC{{O}_{3}}\],\[Ce{{O}_{2}}\], sand | Absorbs ultra violet rays, for making lenses |
| Jena glass | Zinc and Barium Boro silicates | It is resistant to heat shock and common reagent. It is used for making good quality of glass wares. |
Coloured glass : Addition of transition metal compounds to glass give coloured glasses . Small amounts of Cr(III), Mn(IV), Co(II) and Fe(III) compounds impart green, violet blue or brown colour respectively
| Compound added - Colour imparted | Compound added - Colour imparted |
| Cobalt axide ( CoO) - Blue | Chromium oxide (\[C{{r}_{2}}{{O}_{3}}\]) - Green |
| Cuprous oxide (\[C{{u}_{2}}O\]) - Red | Auric chloride \[(AuC{{l}_{3}})\] - Ruby |
| Cadmium sulphide (CdS) - Lemon yellow | Manganese dioxide (\[Mn{{O}_{2}})\] - Purple |
Etching of glass : Glass is attacked by hydrofluoric acid. This property is used in the etching of glass. The glass to be etched is coated with a thin layer of wax and the design to be produced is scratched with a needle. An aqueous solution of \[HF\] is applied to the exposed part. After some time it is placed in hot water and wax is removed from the surface. The marks are engraved on the exposed parts.
Tin and its Compounds
(1) Important ore : Cassiterite (tin stone) \[Sn{{O}_{2}}\]
(2) Extraction of tin from tin stone.
(i) Concentration : The powdered tin stone is concentrated by gravity separation and the magnetic impurities like wolframite etc., are separated from tin stone by magnetic separators.
(ii) Roasting : The concentrated ore is heated in a current of air when impurities like \[S\] and \[As\] are oxidised to volatile \[S{{O}_{2}}\] and \[A{{s}_{2}}{{O}_{3}}\]. Iron pyrites change to their oxides and sulphates.
(iii) Leaching and washing : The roasted ore is treated with water when \[CuS{{O}_{4}}\] and \[FeS{{O}_{4}}\] are washed away from the main ore. Further lighter ferric oxide is washed away leaving behind heavier ore particles known as black tin containing 60 to 70% \[Sn{{O}_{2}}\]. \[Sn{{O}_{2}}+2C\xrightarrow{{}}Sn+2CO\]
\[\underset{\text{Flux}}{\mathop{CaC{{O}_{3}}}}\,\xrightarrow{{}}CaO+C{{O}_{2}}\];
\[CaO+Si{{O}_{2}}\xrightarrow{{}}\underset{\text{Slag}}{\mathop{CaSi{{O}_{3}}}}\,\]
Molten tin is drawn into blocks. It contains 99.5 percent of tin metal and is called block tin.
Refining of tin : It is purified by liquation, poling and electrolytic refining.
For very high purity, it is purified by electrolytic method. The electrolyte consists of tin sulphate containing a small amount of hydrofluorosilicic acid \[({{H}_{2}}Si{{F}_{6}})\] and sulphuric acid. Impure tin makes anode while pure tin sheet serves as cathode.
(3) Comounds of Tin
(i) Stannic oxide, SnO2 : It is prepared by heating tin strongly in air. \[Sn+{{O}_{2}}\xrightarrow{{}}Sn{{O}_{2}}\]
It can also be prepared by heating metastannic acid obtained by the action of conc. \[HN{{O}_{3}}\] on tin.
\[Sn+4HN{{O}_{3}}(conc.)\xrightarrow{{}}{{H}_{2}}Sn{{O}_{3}}+4N{{O}_{2}}+{{H}_{2}}O\]
\[{{H}_{2}}Sn{{O}_{3}}\xrightarrow{\Delta }\,Sn{{O}_{2}}+{{H}_{2}}O\]
It occurs in nature as tin stone. It is a white solid insoluble in water and is amphoteric in nature. With \[NaOH\] it forms \[N{{a}_{2}}Sn{{O}_{3}}\]. It is used for making enamels and glazes for tiles, pottery etc. it is also used as a polishing powder.
(ii) Stannous oxide, SnO : It is prepared by heating stannous oxalate \[Sn{{C}_{2}}{{O}_{4}}\xrightarrow{\Delta }\,SnO+CO+C{{O}_{2}}\]
Oxidation of \[SnO\] to \[Sn{{O}_{2}}\] is checked by \[CO\]. It is a grey solid which oxidises readily to \[Sn{{O}_{2}}\] when heated in air.
\[2SnO+{{O}_{2}}\xrightarrow{{}}2Sn{{O}_{2}}\]
It is amphoteric in nature and reacts both with acids and alkalies. With \[NaOH\] it forms \[N{{a}_{2}}Sn{{O}_{2}}\].
(iii) Stannous sulphide, SnS : It is insoluble in water but soluble in hot conc. \[HCl\]. In yellow ammonium polysulphide it gets converted to ammonium thiostannate \[{{(N{{H}_{4}})}_{2}}Sn{{S}_{3}}\].
(iv) Stannous chloride, SnCl2 : When \[Sn\] is heated with \[HCl\] (conc.) \[SnC{{l}_{2}}\] is formed.
\[Sn(s)+HCl(Conc.)\xrightarrow{{}}\,SnC{{l}_{2}}(aq)+{{H}_{2}}(g)\]
On concentrating the resulting solution, crystals of \[SnC{{l}_{2}}.2{{H}_{2}}O\] are obtained. When it is heated, basic tin chloride is obtained.
\[SnC{{l}_{2}}.2{{H}_{2}}O(s)\xrightarrow{\Delta }\,Sn(OH)Cl+HCl+{{H}_{2}}O\]
To obtain anhydrous \[SnC{{l}_{2}}\], heat \[Sn\] in dry \[HCl\] gas.
\[Sn+2HCl\xrightarrow{\Delta }\,SnC{{l}_{2}}+{{H}_{2}}\]
(a) It exists as a anhydrous (white powder, m.p. = \[520K\], rhombic solid) as well as dihydrate \[SnC{{l}_{2}}.2{{H}_{2}}O\](white, m.p. \[=480K\], monoclinic) and is used as a strong reducing agent in conc. \[HCl\] in laboratory.
\[SnC{{l}_{2}}\] also reduces \[HgC{{l}_{2}}\]
\[2HgC{{l}_{2}}+SnC{{l}_{2}}\xrightarrow{{}}H{{g}_{2}}C{{l}_{2}}+SnC{{l}_{4}}\]
\[H{{g}_{2}}C{{l}_{2}}+SnC{{l}_{2}}\xrightarrow{{}}2Hg+SnC{{l}_{4}}\]
(b) It is precipitated as hydroxide by an alkali.
(c) If forms addition compounds with \[N{{H}_{3}}\] such as \[SnC{{l}_{2}}.N{{H}_{3}}\] and \[SnC{{l}_{2}}.2N{{H}_{3}}\].
(v) Stannic chloride, SnCl4 : It is obtained by the action of \[C{{l}_{2}}\] on molten \[Sn\]
\[Sn+2C{{l}_{2}}\xrightarrow{{}}SnC{{l}_{4}}\]
It can also be obained by distilling tin with mercuric chloride.
\[Sn+2HgC{{l}_{2}}\xrightarrow{{}}SnC{{l}_{4}}+2Hg\]
(a) It is a colourless fuming liquid (b.p \[388K\]) soluble in water.
It is used as a strong reducing agent in laboratory. It is also used as a mordant in dyeing.
(b) It can exist as \[SnC{{l}_{4}}.5{{H}_{2}}O\] and with excess water it is hydrolysed to form basic chloride and ultimately stannic acid \[({{H}_{2}}Sn{{O}_{4}})\].
\[SnC{{l}_{4}}+{{H}_{2}}O\xrightarrow{{}}Sn(OH)Cl+HCl\]
\[Sn(OH)Cl+3{{H}_{2}}O\xrightarrow{{}}Sn{{(OH)}_{4}}\]or \[{{H}_{2}}Sn{{O}_{4}}+3HCl\]
Its hydrolysis is prevented by \[HCl\] which forms complex anion \[{{[SnC{{l}_{6}}]}^{2-}}\]
(c) It forms double salts with \[N{{H}_{3}},{{N}_{2}}O,\,PC{{l}_{5}}\] e.g., \[SnC{{l}_{4}}.4N{{H}_{3}}\].
It is used as a mordant and tinning agent.
(vi) Stannous fluoride, SnF2 : It is obtained by dissolving \[SnO\] in \[HF\]
\[SnO+2HF\xrightarrow{{}}Sn{{F}_{2}}+{{H}_{2}}O\]
It is a white crystalline solid insoluble in water. It is used in tooth pastes to help in controlling dental decay.
Tinning : During cooking, organic acids present in food stuff attack the household utensils made of copper, brass etc. in the presence of air. since tin is not attacked by organic acids, the utensils are protected by tinning.
Lead
(1) Some important ores
Galena; \[-PbS\](Main); Cerussite \[-PbC{{O}_{3}}\]
Anglesite \[-PbS{{O}_{4}}\], lararkite \[PbO.PbS{{O}_{4}}\]
(2) Extraction from galena
(i) Concentration : The finely powdered ore is concentrated by froth floatation process.
(ii) Reduction process
(a) Self reduction process
\[2PbS+3{{O}_{2}}\xrightarrow{\Delta }2PbO+2S{{O}_{2}}\]
\[PbS+2{{O}_{2}}\xrightarrow{\Delta }PbS{{O}_{4}}\]
\[PbS+2PbO\xrightarrow{\Delta }3Pb+S{{O}_{2}}\]
\[PbS+PbS{{O}_{4}}\xrightarrow{\Delta }\,2Pb+2S{{O}_{2}}\]
(b) Carbon reduction process
\[S+{{O}_{2}}\xrightarrow{\Delta }S{{O}_{2}}\]; \[4As+3{{O}_{2}}\xrightarrow{\Delta }2A{{s}_{2}}{{O}_{3}}\]
\[2PbS+3{{O}_{2}}\xrightarrow{\Delta }2PbO+2S{{O}_{2}}\]
\[PbO+C\xrightarrow{\Delta }Pb+CO\]; \[PbO+CO\xrightarrow{\Delta }Pb+C{{O}_{2}}\]
\[\underset{\text{Lime}}{\mathop{CaC{{O}_{3}}}}\,\xrightarrow{\Delta }CaO+C{{O}_{2}}\]; \[CaO+Si{{O}_{2}}\xrightarrow{\Delta }\underset{\text{Slag}}{\mathop{CaSi{{O}_{3}}}}\,\]
(iii) Purification : It is purified electrolytically. The electrolyte consists of lead silicofluoride \[(PbSi{{F}_{6}})\] and hydrofluosilicic acid. Impure lead is made anode and sheet of pure lead serves as cathode.
Properties of Lead
With oxygen lead form oxides, with chlorine it forms chloride \[PbC{{l}_{2}}\], with sulphur it gives sulphide \[PbS\] and with \[{{H}_{2}}S{{O}_{4}}\] the corresponding sulphate \[PbS{{O}_{4}}\]. With \[NaOH\] it forms plumbate.
\[Pb+2NaOH\xrightarrow{{}}N{{a}_{2}}Pb{{O}_{2}}+{{H}_{2}}\]
(3) Compounds of Lead
(i) Lead oxide (Litharge), PbO : It is prepared by heating the nitrate.
\[2Pb{{(N{{O}_{3}})}_{2}}\xrightarrow{\Delta }2PbO+4N{{O}_{2}}+{{O}_{2}}\]
It exist in two varieties yellow form (messicol) and red form (litherage). Yellow form is prepared by gently heating lead in air while fusion yield red form. It is insoluble in water and amphoteric in nature.
It dissolves in \[NaOH\] to form sod. Plumbite.
\[PbO+2NaOH\xrightarrow{{}}N{{a}_{2}}Pb{{O}_{2}}+{{H}_{2}}O\]
It can be reduced with various reducing agents (\[C,\,{{H}_{2}},CO\] etc.) to lead.
It is used in paints and varnishes, for making flint glass, for making lead (II) salts and for glazing pottery.
(ii) Lead dioxide, PbO2 : It is prepared by heating \[P{{b}_{3}}{{O}_{4}}(2PbO+Pb{{O}_{2}})\] with dilute \[HN{{O}_{3}}\]
\[P{{b}_{3}}{{O}_{4}}+4HN{{O}_{3}}\xrightarrow{\Delta }2Pb{{(N{{O}_{3}})}_{2}}+2{{H}_{2}}O+Pb{{O}_{2}}\]
It is amphoteric in nature and dissolve in \[NaOH\] to form sodium plumbate.
\[2NaOH+Pb{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}Pb{{O}_{3}}+{{H}_{2}}O\]
It is a powerful oxidising agent. It reacts with conc. \[HCl\] on warming to give \[PbC{{l}_{4}}\]
\[Pb{{O}_{2}}+4HCl\xrightarrow{Warm}PbC{{l}_{4}}+{{H}_{2}}O\]
It is a chocolate brown solid insoluble in water and nitric acid. It is a powerful oxidizing agent. It is amphoteric in nature and is used in lead storage batteries and in safety matches.
(iii) Minium or sindhur or Red lead, Pb3O4 : It is prepared by heating \[PbO\] in air to above \[673K\].
\[6PbO+{{O}_{2}}\xrightarrow{673K}2P{{b}_{3}}{{O}_{4}}\]
It is a red crystalline solid insoluble in water.
It is a mixed oxide \[Pb{{O}_{2}}+2PbO\] and reacts with \[HN{{O}_{3}}\] to form \[Pb{{(N{{O}_{3}})}_{2}}\] and \[Pb{{O}_{2}}\]
\[2PbO.Pb{{O}_{2}}+4HN{{O}_{3}}\to Pb{{(N{{O}_{3}})}_{2}}+2{{H}_{2}}O+Pb{{O}_{2}}\]
It is a strong oxidising agent. It liberates chlorine with conc. \[HCl\] and \[{{O}_{2}}\] with conc. \[{{H}_{2}}S{{O}_{4}}\]
\[P{{b}_{3}}{{O}_{4}}+8HCl\xrightarrow{{}}3PbC{{l}_{2}}+4{{H}_{2}}O+C{{l}_{2}}\]
\[2P{{b}_{3}}{{O}_{4}}+6{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}6PbS{{O}_{4}}+6{{H}_{2}}O+{{O}_{2}}\]
It is used as a protective paint in iron, steel and silver mirrors and in glass industry.
(iv) Lead chloride, PbCl2 : It can be prepared by treating a salt of lead with dil. \[HCl\]
\[Pb{{(N{{O}_{3}})}_{2}}+2HCl\xrightarrow{{}}PbC{{l}_{2}}+2HN{{O}_{3}}\]
It can also be obtained by dissolving lead (II) oxide to lead (II) carbonate in \[HCl\]. It is soluble in hot water but precipitate out in cold water. it is soluble in conc. \[HCl\] due to the formation of a complex, tetrachloroplumbate (II) ion.
\[PbC{{l}_{2}}+2HCl\xrightarrow{{}}{{H}_{2}}[PbC{{l}_{4}}]\]
It also reacts with hot lime water to give \[Pb(OH)Cl\] which is used as white pigment.
\[PbC{{l}_{2}}+Ca{{(OH)}_{2}}\xrightarrow{{}}Pb(OH)Cl+CaO+HCl\]
(v) Lead tetrachloride, PbCl4 : It is obtained by heating of \[Pb{{O}_{2}}\] with conc. \[HCl\].
It is a yellow oily fuming liquid which decomposes into \[PbC{{l}_{2}}\] at \[373K\].
\[PbC{{l}_{4}}\xrightarrow{373K}PbC{{l}_{2}}+C{{l}_{2}}\]
It also combines with \[HCl\] to form complex hexachloroplumbate (IV) ion.
\[PbC{{l}_{4}}+2HCl\xrightarrow{{}}{{H}_{2}}[PbC{{l}_{6}}]\]
You need to login to perform this action.
You will be redirected in
3 sec
