Physico-Chemical Properties Of d-Block Elements
Category : JEE Main & Advanced
(1) Atomic radii : The atomic, radii of 3d-series of elements are compared with those of the neighbouring s and p-block elements.
| K | Ca | Sc | Ti | V | Cr | Mn |
| 227 | 197 | 144 | 132 | 122 | 117 | 117 |
| Fe | Co | Ni | Cu | Zn | Ga | Ge |
| 117 | 116 | 115 | 117 | 125 | 135 | 122* |
The atomic radii of transition elements show the following characteristics,
(i) The atomic radii and atomic volumes of d-block elements in any series decrease with increase in the atomic number. The decrease however, is not regular. The atomic radii tend to reach minimum near at the middle of the series, and increase slightly towards the end of the series.
Explanation : When we go in any transition series from left, to right, the nuclear charge increases gradually by one unit at each elements. The added electrons enter the same penultimate shell, (inner d-shell). These added electrons shield the outermost electrons from the attraction of the nuclear charge. The increased nuclear charge tends to reduce the atomic radii, while the added electrons tend to increase the atomic radii. At the beginning of the series, due to smaller number of electrons in the d-orbitals, the effect of increased nuclear charge predominates, and the atomic radii decrease. Later in the series, when the number of d-electrons increases, the increased shielding effect and the increased repulsion between the electrons tend to increase the atomic radii. Somewhere in the middle of the series, therefore the atomic radii tend to have a minimum value as observed.
(ii) The atomic radii increase while going down in each group. However, in the third transition series from hafnium (Hf) and onwards, the elements have atomic radii nearly equal to those of the second transition elements.
Explanation : The atomic radii increase while going down the group. This is due to the introduction of an additional shell at each new element down the group. Nearly equal radii of second and third transition series elements is due to a special effect called lanthanide contraction.
(2) Ionic radii : For ions having identical charges, the ionic radii decrease slowly with the increase in the atomic number across a given series of the transition elements.
| Elements (m): | Ionic radius,(M2+)/pm: | Pm:(M3+)/pm: |
| Sc | – | 81 |
| Ti | 90 | 76 |
| V | 88 | 74 |
| Cr | 84 | 69 |
| Mn | 80 | 66 |
| Fe | 76 | 64 |
| Co | 74 | 63 |
| Ni | 72 | – |
| Cu | 69 | – |
| Zn | 74 | – |
Explanation : The gradual decrease in the values of ionic radius across the series of transition elements is due to the increase in the effective nuclear charge.
(3) Ionisation energies : The ionisation energies of the elements of first transition series are given below:
| Elements | I1 | I2 | I3 |
| Sc | 632 | 1245 | 2450 |
| Ti | 659 | 1320 | 2721 |
| V | 650 | 1376 | 2873 |
| Cr | 652 | 1635 | 2994 |
| Mn | 716 | 1513 | 3258 |
| Fe | 762 | 1563 | 2963 |
| Co | 758 | 1647 | 3237 |
| Ni | 736 | 1756 | 3400 |
| Cu | 744 | 1961 | 3560 |
| Zn | 906 | 1736 | 3838 |
* in kJ mol–1
The following generalizations can be obtained from the ionisation energy values given above.
(i) The ionisation energies of these elements are high and in the most cases lie between those of s- and p-block elements. This indicates that the transition elements are less electropositive than s-block elements.
Explanation : Transition metals have smaller atomic radii and higher nuclear charge as compared to the alkali metals. Both these factors tend to increase the ionisation energy, as observed.
(ii) The ionisation energy in any transition series increases in the nuclear with atomic number; the increase however is not smooth and as sharp as seen in the case of s and p-block elements.
Explanation : The ionisation energy increases due to the increase in the nuclear charge with atomic number at the beginning of the series. Gradually, the shielding effect of the added electrons also increases. This shielding effect tends to decrease the attraction due to the nuclear charge. These two opposing factors lead to a rather gradual increase in the ionisation energies in any transition series.
(iii) The first ionisation energies of 5d-series of elements are much higher than those of the 3d and 4d series elements.
Explanation : In the 5d-series of transitions elements, after lanthanum (La), the added electrons go to the next inner 4f orbitals. The 4f electrons have poor shielding effect. As a result, the outermost electrons experience greater nuclear attraction. This leads to higher ionisation energies for the 5d- series of transition elements.
(4) Metallic character : All the transition elements are metals. These are hard, and good conductor of heat and electricity. All these metals are malleable, ductile and form alloys with other metals. These elements occur in three types e.g., face- centered cubic (fcc), hexagonal close-packed (hcp) and body-centered cubic (bcc), structures.
The transition elements shows both covalent as well as metallic bonding amongst their atoms.
Explanation : The ionisation energies of the transition elements are not very high. The outermost shell in their atoms have many vacant, partially filled orbitals. These characteristics make these elements metallic in character. The hardness of these metals, suggests the presence of covalent bonding in these metals. The presence of unfilled d-orbitals favour covalent bonding. Metallic bonding in these metals is indicated by the conducting nature of these metals. Therefore, it appears that there exists covalent and metallic bonding in transition elements.
(5) Melting and boiling points : The melting and boiling points of transition elements except Cd and Hg, are very high as compared to the s-block and p-block elements. The melting and boiling points first increase, pass through maxima and then steadily decrease across any transition series. The maximum occurs around middle of the series.
Explanation : Atoms of the transition elements are closely packed and held together by strong metallic bonds which have appreciable covalent character. This leads to high melting and boiling points of the transition elements.
The strength of the metallic bonds depends upon the number of unpaired electrons in the outermost shell of the atom. Thus, greater is the number of unpaired electrons stronger is the metallic bonding. In any transition element series, the number of unpaired electrons first increases from 1 to 5 and then decreases back to the zero .The maximum five unpaired electrons occur at Cr (3d series). As a result, the melting and boiling points first increase and then decrease showing maxima around the middle of the series.
The low melting points of Zn, Cd, and Hg may be due to the absence of unpaired d-electrons in their atoms.
(6) Enthalpies of atomization : Transition metals exhibit high enthalpies of atomization.
Explanation : This is because the atoms in these elements are closely packed and held together by strong metallic bonds. The metallic bond is formed as a result of the interaction of electrons in the outermost shell. Greater the number of valence electrons, stronger is the metallic bond.
(7) Oxidation states : Most of the transition elements exhibit several oxidation states i.e., they show variable valency in their compounds. Some common oxidation states of the first transition series elements are given below in table,
Outer Ele. Confi. and O. S. for 3d- elements
| Elements | Outer electronic configuration | Oxidation states |
| Sc | \[3{{d}^{1}}4{{s}^{2}}\] | + 2, + 3 |
| Ti | \[3{{d}^{2}}4{{s}^{2}}\] | + 2, + 3, + 4 |
| V | \[3{{d}^{3}}4{{s}^{2}}\] | + 2,+ 3,+ 4,+ 5 |
| Cr | \[3{{d}^{5}}4{{s}^{1}}\] | + 1, + 2, + 3, + 4, + 5, + 6 |
| Mn | \[3{{d}^{5}}4{{s}^{2}}\] | + 2, + 3, + 4, + 5, + 6, + 7 |
| Fe | \[3{{d}^{6}}4{{s}^{2}}\] | + 2, + 3, + 4, + 5, + 6 |
| Co | \[3{{d}^{7}}4{{s}^{2}}\] | + 2, + 3, + 4 |
| Ni | \[3{{d}^{8}}4{{s}^{2}}\] | + 2, + 3, + 4 |
| Cu | \[3{{d}^{10}}4{{s}^{1}}\] | + 1,+ 2 |
| Zn | \[3{{d}^{10}}4{{s}^{2}}\] | + 2 |
Explanation : The outermost electronic configuration of the transition elements is \[(n-1){{d}^{1-10}}n{{s}^{2}}\]. Since, the energy levels of \[(n-1)d\] and ns-orbitals are quite close to each other, hence both the ns and \[(n-1)\] d-electrons are available for bonding purposes. Therefore, the number of oxidation states show by these elements depends upon the number of d-electrons it has. For example, Sc having a configuration \[3{{d}^{1}}4{{s}^{2}}\] may show an oxidation state of + 2 (only s-electrons are lost) and + 3 (when d-electron is also lost). The highest oxidation state which an elements of this group might show is given by the total number of ns and \[(n-1)\] d-electrons.
The relative stability of the different oxidation states depends upon the factors such as, electronic configuration, nature of bonding, stoichiometry, lattice energies and solvation energies. The highest oxidation states are found in fluorides and oxides because fluorine and oxygen are the most electronegative elements. The highest oxidation state shown by any transition metal is eight. The oxidation state of eight is shown by Ru and Os.
An examination of the common oxidation states reveals the following conclusions.
(i) The variable oxidation states shown by the transition elements are due to the participation of outer ns and inner \[(n-1)\] d-electrons in bonding.
(ii) Except scandium, the most common oxidation state shown by the elements of first transition series is +2. This oxidation state arises from the loss of two 4s electrons. This means that after scandium, d-orbitals become more stable than the s-orbital.
(iii) The highest oxidation states are observed in fluorides and oxides. The highest oxidation state shown by any transition elements (by Ru and Os) is 8.
(iv) The transition elements in the + 2 and + 3 oxidation states mostly form ionic bonds. In compounds of the higher oxidation states (compound formed with fluorine or oxygen), the bonds are essentially covalent. For example, in permanganate ion \[MnO_{4}^{-}\], all bonds formed between manganese and oxygen are covalent.
(v) Within a group, the maximum oxidation state increases with atomic number. For example, iron shown the common oxidation state of + 2 and + 3, but ruthenium and osmium in the same group form compounds in the + 4, + 6 and + 8 oxidation states.
(vi) Transition metals also form compounds in low oxidation states such as +1 and 0. For example, nickle in, nickel tetracarbonyl, \[Ni{{(CO)}_{4}}\] has zero oxidation state. Similarly Fe in \[(Fe{{(CO)}_{5}}\] has zero oxidation state.
The bonding in the compounds of transition metals in low oxidation states is not always very simple.
(vii) Ionisation energies and the stability of oxidation states :The values of the ionisation energies can be used in estimating the relative stability of various transition metal compounds (or ions). For example, \[N{{i}^{2+}}\] compounds are found to be thermodynamically more stable than \[P{{t}^{2+}},\] whereas \[P{{t}^{4+}}\] compounds are more stable than \[N{{i}^{4+}}\] compounds. The relative stabilities of \[N{{i}^{2+}}\] relative to \[P{{t}^{2+}}\] and that of \[P{{t}^{4+}}\] relative to \[N{{i}^{4+}}\] can be explained as follows,
The first four ionisation energies of Ni and Pt
| Metal | \[\left( I{{E}_{1}}+I{{E}_{2}} \right)kJmo{{l}^{-1}},\] | \[\left( I{{E}_{3}}+I{{E}_{4}} \right)~kJmo{{l}^{-1}},\] |
Etotal, \[kJ\text{ }mo{{l}^{-1}}\] \[\left( =I{{E}_{1}}+I{{E}_{2}}+I{{E}_{3}}+I{{E}_{4}} \right)\] |
| Ni | 2490 | 8800 | 11290 |
| Pt | 2660 | 6700 | 9360 |
Thus, the ionisation of Ni to \[N{{i}^{2+}}\] requires lesser energy (2490 kJ \[mo{{l}^{1}}\]) as compared to the energy required for the production of \[P{{t}^{2+}}\] (2660 kJ\[mo{{l}^{1}}\]). Therefore, \[N{{i}^{2+}}\] compounds are thermodynamically more stable than \[P{{t}^{2+}}\] compounds.
On the other hand, formation of \[P{{t}^{4+}}\] requires lesser energy (9360 kJ \[mo{{l}^{1}}\]) as compared to that required for the formation of \[N{{i}^{4+}}\] (11290 kJ \[mo{{l}^{1}}\]). Therefore, \[P{{t}^{4+}}\] compounds are more stable than \[N{{i}^{4+}}\] compounds.
This is supported by the fact that \[{{\left[ PtC{{l}_{6}} \right]}^{2}}\] complex ion is known, while the corresponding ion for nickel is not known. However, other factors which affect the stability of a compound are,
(a) Enthalpy of sublimation of the metal.
(b) Lattice and the solvation energies of the compound or ion.
(viii) Transition elements like Sc, Y, La and Ac do not show variable valency.
(8) Electrode potentials \[({{E}^{o}})\]: Standard electrode potentials of some half–cells involving 3d-series of transition elements and their ions in aqueous solution are given in table,
Standard electrode potentials for 3d-elements
| Elements | Ion | Electrode reaction | \[{{E}^{o}}\]/ volt |
| Sc | \[S{{c}^{3+}}\] | \[S{{c}^{3+}}+~3{{e}^{}}\to Sc\] | – 2.10 |
| Ti | \[T{{i}^{2+}}\] | \[T{{i}^{2+}}+~2{{e}^{}}\to Ti\] | – 1.60 |
| V | \[{{V}^{2+}}\] | \[{{V}^{2+}}+~2{{e}^{}}\to V\] | – 1.20 |
| Cr | \[C{{r}^{3+}}\] | \[C{{r}^{3+}}+~3{{e}^{}}\to Cr\] | – 0.71 |
| Mn | \[M{{n}^{2+}}\] | \[M{{n}^{2+}}+~2{{e}^{}}\to Mn\] | – 1.18 |
| Fe | \[F{{e}^{2+}}\] | \[F{{e}^{2+}}+~2{{e}^{}}\to Fe\] | – 0.44 |
| Co | \[C{{o}^{2+}}\] | \[C{{o}^{2+}}+~2{{e}^{}}\to Co\] | – 0.28 |
| Ni | \[N{{i}^{2+}}\] | \[N{{i}^{2+}}+~2{{e}^{}}\to Ni\] | – 0.24 |
| Cu | \[C{{u}^{2+}}\] | \[C{{u}^{2+}}+~2{{e}^{}}\to Cu\] | + 0.34 |
| Zn | \[Z{{n}^{2+}}\] | \[Z{{n}^{2+}}+2{{e}^{}}\to Zn\] | – 0.76 |
The negative values of \[E{}^\circ \] for the first series of transition elements (except for \[C{{u}^{2+}}/\text{ }Cu\]) indicate that,
(i) These metals should liberate hydrogen from dilute acids i.e., the reactions, \[M~+~2{{H}^{+}}\to {{M}^{2+}}+{{H}_{2}}(g);\,\,\,2M+6{{H}^{+}}\to \,\,2{{M}^{3+}}+3{{H}_{2}}(g)\] are favourable in the forward direction. In actual practice however, most of these metals react with dilute acids very slowly. Some of these metals get coated with a thin protective layer of oxide. Such an oxide layer prevents the metal to react further.
(ii) These metals should act as good reducing agents. There is no regular trend in the \[{{E}^{o}}\] values. This is due to irregular variation in the ionisation and sublimation energies across the series.
Relative stabilities of transition metal ions in different oxidation states in aqueous medium can be predicted from the electrode potential data. To illustrate this, let us consider the following,
\[M(s)\to \,M(g)\] ; \[\Delta {{H}_{1}}=\] Enthalpy of sublimation, \[\Delta {{H}_{sub}}\]
\[M(g)\to {{M}^{+}}(g)+{{e}^{-}}\] ; \[\Delta {{H}_{2}}=\text{Ionisation energy},IE\]
\[{{M}^{+}}(g)\to \,{{M}^{+}}(aq)\] ; \[\Delta {{H}_{3}}\,=\,\text{Enthalpy of hydration,}\Delta {{H}_{hyd}}\,\,\]
Adding these equations one gets,
\[M(s)\to {{M}^{+}}(aq)+{{e}^{-}}\]
\[\Delta H=\Delta {{H}_{1}}+\Delta {{H}_{2}}+\Delta {{H}_{3}}=\Delta {{H}_{sub}}+IE+\Delta {{H}_{hyd}}\]
The \[\Delta H\]represents the enthalpy change required to bring the solid metal M to the monovalent ion in aqueous medium, \[{{M}^{+}}(aq)\].
The reaction, \[M(s)\to {{M}^{+}}(aq)+{{e}^{-}},\] will be favourable only if \[\Delta H\]is negative. More negative is the value is of \[\Delta H\], more favourable will be the formation of that cation from the metal. Thus, the oxidation state for which \[\Delta H\] value is more negative will be stable in the solution.
Electrode potential for a \[{{M}^{n+}}/M\] half-cell is a measure of the tendency for the reaction, \[{{M}^{n+}}(aq)+n{{e}^{-}}\to M(s)\]
Thus, this reduction reaction will take place if the electrode potential for \[{{M}^{n+}}/M\] half-cell is positive. The reverse reaction, \[M(s)\to {{M}^{n+}}(aq)+n{{e}^{-}}\]
Involving the formation of \[M{{n}^{+}}(aq)\] will occur if the electrode potential is negative, i.e., the tendency for the formation of\[{{M}^{n+}}(aq)\] from the metal M will be more if the corresponding \[{{E}^{o}}\] value is more negative. In other words, the oxidation state for which \[{{E}^{o}}\] value is more negative (or less positive) will be more stable in the solution.
When an elements exists in more than one oxidation states, the standard electrode potential \[({{E}^{o}})\] values can be used in the predicting the relative stabilities of different oxidation states in aqueous solutions. The following rule is found useful.
The oxidation state of a cation for which \[\Delta H=(\Delta {{H}_{sub}}+lE\,+\Delta {{H}_{hyd}})\]or \[{{E}^{o}}\] is more negative (for less positive) will be more stable.
(9) Formation of coloured ions : Most of the compound of the transition elements are coloured in the solid state and /or in the solution phase. The compounds of transition metals are coloured due to the presence of unpaired electrons in their d-orbitals.
Explanation : In an isolated atom or ion of a transition elements, all the five d-orbitals are of the same energy (they are said to be regenerate). Under the influence of the combining anion (s), or electron- rich molecules, the five d-orbitals split into two (or sometimes more than two) levels of different energies. The difference between the two energy levels depends upon the nature of the combining ions, but corresponds to the energy associated with the radiations in the visible region, \[(\lambda =380-760nm)\]. Typical splitting for octahedral and tetrahedral geometries are shown in fig.
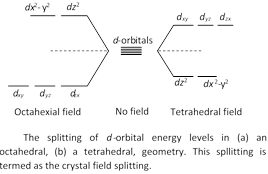
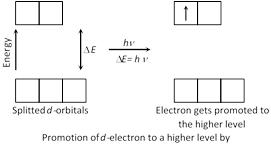
The transition metals in elements form or in the ionic form have one or more unpaired electrons. When visible light falls on the sample, the electrons from the lower energy level get promoted to a higher energy level due to the absorption of light of a characteristic wavelength (or colour). This wavelength (or colour) of the absorbed light depends upon the energy difference of the two levels. Rest of the light gets transmitted. The transmitted light has a colour complementary to the absorbed colour. Therefore, the compound or the solution appears to be of the complementary colour. For example, \[Cu({{H}_{2}}O)_{6}^{2+}\] ions absorb red radiation, and appear blue-green (blue-green is complementary colour to red). Hydrated \[C{{o}^{2+}}\] ions absorb radiation in the blue-green region, and therefore, appear red in sunlight. Relationship between the colour of the absorbed radiation and that of the transmitted light is given in table
Relationship between the colours of the absorbed and transmitted light: the complementary colours.
| Colour of the | Colour of the | ||
| Absorbed light | Transmitted light | Absorbed light | Transmitted light |
| IR | White | Blue-green | Red |
| Red | Blue-green | Blue | Orange |
| Orange | Blue | Indigo | Yellow |
| Yellow | Indigo | Violet | Yellow-green |
| Yellow-green | Violet | UV | White |
| Green | Purple | ||
However, if radiations of all the wavelengths (or colours) except one are absorbed, then the colour of the substance will be the colour of the transmitted radiation. For example, if a substance absorbs all colours except green, then it would appear green to the eyes.
The transition metal ions which have completely filled d-orbitals are colourless, as there are no vacant d-orbitals to permit promotion of the electrons. Therefore, \[Z{{n}^{2+}}(3{{d}^{10}}),\,C{{d}^{2}}+(4{{d}^{10}})\] and \[H{{g}^{2+}}\,(5{{d}^{10}})\,\,S{{c}^{3+}},T{{i}^{4+}},C{{u}^{+}}\] ions and Zn, Cd, Hg are colourless and diamagnetic. The transition metal ions which have completely empty d-orbitals are also colourless, Thus, \[S{{c}^{3+}}\]and \[T{{i}^{4+}}\] ions are colourless, unless a coloured anion is present in the compound.
Colours and the outer- electronic configurations of the some important ions of the first transition series elements are given bellow,
| Ion | Outer configuration | Number of unpaired electrons | Colour of the ion |
| \[S{{c}^{3+}}\] | \[3{{d}^{0}}\] | 0 | Colourless |
| \[T{{i}^{3+}}\] | \[3{{d}^{1}}\] | 1 | Purple |
| \[T{{i}^{4+}}\] | \[3{{d}^{0}}\] | 0 | Colourless |
| \[{{V}^{3+}}\] | \[3{{d}^{2}}\] | 2 | Green |
| \[C{{r}^{3+}}\] | \[3{{d}^{3}}\] | 3 | Violet |
| \[M{{n}^{2+}}\] | \[3{{d}^{5}}\] | 5 | Light pink |
| \[M{{n}^{3+}}\] | \[3{{d}^{4}}\] | 4 | Violet |
| \[F{{e}^{2+}}\] | \[3{{d}^{6}}\] | 4 | Green |
| \[F{{e}^{3+}}\] | \[3{{d}^{5}}\] | 5 | Yellow |
| \[C{{o}^{3+}}\] | \[3{{d}^{7}}\] | 3 | Pink |
| \[N{{i}^{2+}}\] | \[3{{d}^{8}}\] | 2 | Green |
| \[C{{u}^{2+}}\] | \[3{{d}^{9}}\] | 1 | Blue |
| \[C{{u}^{+}}\] | \[3{{d}^{10}}\] | 0 | Colourless |
| \[Z{{n}^{2+}}\] | \[3{{d}^{10}}\] | 0 | Colourless |
(10) Magnetic properties : Most of the transition elements and their compounds show paramagnetism. The paramagnetism first increases in any transition element series, and then decreases. The maximum paramagnetism is seen around the middle of the series. The paramagnetism is described in Bohr Magneton (BM) units. The paramagnetic moments of some common ions of first transition series are given below in Table
Explanation : A substance which is attracted by magnetic filed is called paramagnetic substance. The substances which are repelled by magnetic filed are, called diamagnetic substances. Paramagnetism is due to the presence of unpaired electrons in atoms, ions or molecules.
The magnetic moment of any transition element or its compound/ion is given by (assuming no contribution from the orbital magnetic moment).
\[{{\mu }_{s}}\,=\sqrt{4S\left( S+1 \right)}\,\,\,\,\,\,\,\,\,\,\,BM=\sqrt{n\left( n+2 \right)}\,\,\,\,\,BM\,\,\]
where, S is the total spin (n\[\times s)\]: n is the number of unpaired electrons and s is equal to ½ (representing the spin of an unpaired electron).
From the equation given above, the magnetic moment \[({{\mu }_{s}})\] increases with an increase in the number of unpaired electrons.
Magnetic moments of some ions of the 3d-series elements
| Ion | Outer configuration | No. of unpaired electrons | Magnetic moment (BM) | |
| Calculated | observed | |||
| \[S{{c}^{3+}}\] | \[3{{d}^{0}}\] | 0 | 0 | 0 |
| \[T{{i}^{3+}}\] | \[3{{d}^{1}}\] | 1 | 1.73 | 1.75 |
| \[T{{i}^{2+}}\] | \[3{{d}^{2}}\] | 2 | 2.84 | 2.86 |
| \[{{V}^{2+}}\] | \[3{{d}^{3}}\] | 3 | 3.87 | 3.86 |
| \[C{{r}^{2+}}\] | \[3{{d}^{4}}\] | 4 | 4.90 | 4.80 |
| \[M{{n}^{2+}}\] | \[3{{d}^{5}}\] | 5 | 5.92 | 5.95 |
| \[F{{e}^{2+}}\] | \[3{{d}^{6}}\] | 4 | 4.90 | 5.0-5.5 |
| \[C{{o}^{2+}}\] | \[3{{d}^{7}}\] | 3 | 3.87 | 4.4-5.2 |
| \[N{{i}^{2+}}\] | \[3{{d}^{8}}\] | 2 | 2.84 | 2.9-3.4 |
| \[C{{u}^{2+}}\] | \[3{{d}^{9}}\] | 1 | 1.73 | 1.4-2.2 |
| \[Z{{n}^{2+}}\] | \[3{{d}^{10}}\] | 0 | 0 | 0 |
In d-obitals belonging to a particular energy level, there can be at the maximum five unpaired electrons in \[{{d}^{5}}\] cases. Therefore, paramagnetism in any transition series first increases, reaches a maximum value for \[{{d}^{5}}\]cases and then decreases thereafter.
(11) Formation of complex ions : Transition metals and their ions show strong tendency for complex formation. The cations of transition elements (d-block elements) form complex ions with certain molecules containing one or more lone-pairs of electrons, viz., CO, NO, \[N{{H}_{3}}\] etc., or with anions such as, \[{{F}^{}},\text{ }C{{l}^{}},\text{ }C{{N}^{}}\] etc. A few typical complex ions are,
\[{{[Fe{{(CN)}_{6}}]}^{4-}},\ {{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}},\,{{[Y{{({{H}_{2}}O)}_{6}}]}^{2+}}\],
\[\,[Ni{{(CO)}_{4}}],\,{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}{{[Fe{{F}_{6}}]}^{3-}}\]
Explanation : This complex formation tendency is due to,
(i) Small size and high nuclear charge of the transition metal cations.
(ii) The availability to vacant inner d-orbitals of suitable energy.
(12) Formation of interstitial compounds : Transition elements form a few interstitial compounds with elements having small atomic radii, such as hydrogen, boron, carbon and nitrogen. The small atoms of these elements get entrapped in between the void spaces (called interstices) of the metal lattice. Some characteristics of the interstitial compound are,
(i) These are non-stoichiometric compounds and cannot be given definite formulae.
(ii) These compounds show essentially the same chemical properties as the parent metals, but differ in physical properties such as density and hardness. Steel and cast iron are hard due to the formation of interstitial compound with carbon. Some non-stoichimetric compounds are, \[VS{{e}_{0.98}}\] (Vanadium selenide), \[F{{e}_{0.94}}O\] and titanium nitride.
Explanation : Interstital compounds are hared and dense. This is because, the smaller atoms of lighter elements occupy the interstices in the lattice, leading to a more closely packed structure. Due to greater electronic interactions, the strength of the metallic bonds also increases.
(13) Catalytic properties : Most of the transition metals and their compounds particularly oxides have good catalytic properties. Platinum, iron, vanadium pentoxide, nickel, etc., are important catalysts. Platinum is a general catalyst. Nickel powder is a good catalyst for hydrogenation of unsaturated organic compound such as, hydrogenation of oils some typical industrial catalysts are,
(i) Vanadium pentoxide \[({{V}_{2}}{{O}_{5}})\] is used in the Contact process for the manufacture of sulphuric acid,
(ii) Finely divided iron is used in the Haber’s process for the synthesis of ammonia.
Explanation : Most transition elements act as good catalyst because of,
(i) The presence of vacant d-orbitals.
(ii) The tendency to exhibit variable oxidation states.
(iii) The tendency to form reaction intermediates with reactants.
(iv) The presence of defects in their crystal lattices.
(14) Alloy formation : Transition metals form alloys among themselves. The alloys of transition metals are hard and high metals are high melting as compared to the host metal. Various steels are alloys of iron with metals such as chromium, vanadium, molybdenum, tungsten, manganese etc.
Explanation : The atomic radii of the transition elements in any series are not much different from each other. As a result, they can very easily replace each other in the lattice and form solid solutions over an appreciable composition range. Such solid solutions are called alloys.
(15) Chemical reactivity : The d-block elements (transition elements) have lesser tendency to react, i.e., these are less reactive as compared to s-block elements.
Explanation : Low reactivity of transition elements is due to,
(i) Their high ionisation energies.
(ii) Low heats of hydration of their ions.
(iii) Their high heats of sublimation.
You need to login to perform this action.
You will be redirected in
3 sec
