Catalyst and Catalysis
Category : NEET
Catalyst and Catalysis Catalysis.
Catalysis.
“Catalyst is a substance which speeds up and speeds down a chemical reaction without itself being used up.”
‘or’
“A catalyst is a foreign substance the addition of which into the reaction mixture accelerates or retards the reaction.”
Types of catalysis.
Catalytic reactions can be broadly divided into the following types,
(1) Homogeneous catalysis : When the reactants and the catalyst are in the same phase (i.e. solid, liquid or gas). The catalysis is said to be homogeneous. The following are some of the examples of homogeneous catalysis.
(i) Oxidation of sulphur dioxide into sulphur trioxide with oxygen in the presence of oxides of nitrogen as the catalyst in the lead chamber process. \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{NO(g)}2S{{O}_{3}}(g)\]
The reactants, products and catalyst all are in gaseous state i.e. same phase.
(ii) Hydrolysis of methyl acetate is catalysed by H+ ions furnished by hydrochloric acid .
\[C{{H}_{3}}COOC{{H}_{3}}(l)+{{H}_{2}}O(l)\xrightarrow{HCl(l)}C{{H}_{3}}COOH(l)+C{{H}_{3}}OH(l)\]
(iii) Hydrolysis of sugar is catalysed by H+ ions furnished by sulphuric acid.
\[{{C}_{12}}{{H}_{22}}{{O}_{11}}(l)+{{H}_{2}}O(l)\xrightarrow{{{H}_{2}}S{{O}_{4}}(l)}{{C}_{6}}{{H}_{12}}{{O}_{6}}(l)+{{C}_{6}}{{H}_{12}}{{O}_{6}}(l)\]
(2) Heterogeneous catalysis : The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis. Some of the examples of heterogeneous catalysis are given below.
(i) Oxidation of sulphur dioxide into sulphur trioxide in the presence of platinum metal or vanadium pentaoxide as catalyst in the contact process for the manufacture of sulphuric acid. The reactants are in gaseous state while the catalyst is in solid state. \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{Pt(s)}2S{{O}_{3}}(g)\]
(ii) Combination between nitrogen and hydrogen to form ammonia in the presence of finely divided iron in Haber’s process.
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow{Fe(s)}2N{{H}_{3}}(g)\]
(iii) Oxidation of ammonia into nitric oxide in the presence of platinum gauze as a catalyst in Ostwald’s process.
\[4N{{H}_{3}}(g)+5{{O}_{2}}(g)\xrightarrow{Pt(s)}4NO(g)+6{{H}_{2}}O(g)\]
(iv) Hydrogenation of vegetable oils in the presence of finely divided nickel as catalyst.
\[\text{Vagetable}\,\text{oils}(l)+{{H}_{2}}(g)\xrightarrow{Ni(s)}\text{Vegetable}\,\text{Ghee}(g)\]
(3) Positive catalysis : When the rate of the reaction is accelerated by the foreign substance, it is said to be a positive catalyst and phenomenon as positive catalysis. Some examples of positive catalysis are given below.
(i) Decomposition of \[{{H}_{2}}{{O}_{2}}\]in presence of colloidal platinum. \[2{{H}_{2}}{{O}_{2}}(l)\xrightarrow{Pt}2{{H}_{2}}O(l)+{{O}_{2}}(g)\]
(ii) Decomposition of \[KCl{{O}_{3}}\]in presence of manganese dioxide. \[2KCl{{O}_{3}}(s)\underset{{{270}^{o}}C}{\mathop{\xrightarrow{Mn{{O}_{2}}(s)}}}\,2KCl(s)+3{{O}_{2}}(g)\]
(iii) Oxidation of ammonia in presence of platinum gauze. \[4N{{H}_{3}}(g)+5{{O}_{2}}(g)\underset{{{300}^{o}}C}{\mathop{\xrightarrow{Pt(s)}}}\,4NO(g)+6{{H}_{ & 2}}O(g)\]
(iv) Oxidation of sulphur dioxide in presence of nitric oxide. \[2S{{O}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{NO(g)}2S{{O}_{3}}(g)\]
(v) Oxidation of sulphur dioxide in presence of platinised asbestos or vanadium pentaoxide.
\[2S{{O}_{2}}(g)+{{O}_{2}}(g)\underset{or\,Pt(s)}{\mathop{\xrightarrow{{{V}_{2}}{{O}_{5}}(s)}}}\,2S{{O}_{3}}(g)\]
(vi) Oxidation of hydrochloric acid into chlorine by Deacon’s process in presence of \[CuC{{l}_{2}}\].
\[4HCl(g)+{{O}_{2}}(g)\underset{{{450}^{o}}C}{\mathop{\xrightarrow{CuC{{l}_{2}}(s)}}}\,2C{{l}_{2}}(g)+2{{H}_{2}}O(g)\]
(vii) Formation of methane in presence of nickel.\[CO(g)+3{{H}_{2}}(g)\xrightarrow{Ni(s)}C{{H}_{4}}(g)+{{H}_{2}}O(g)\]
(viii) Synthesis of ammonia by Haber’s process in presence of a mixture of iron and molybdenum.
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\underset{450-{{500}^{o}}C}{\mathop{\xrightarrow{Fe(s)\,\And \,Mo(s)}}}\,2N{{H}_{3}}(g)\]
(ix) Hydrogenation of vegetable oil in presence of nickel.\[\text{Vegetable}\,\text{oil}\ (l)\,+{{H}_{2}}(g)\xrightarrow{Ni(S)}\text{Ghee}(s)\]
(x) Manufacture of methyl alcohol in presence of \[ZnO/C{{r}_{2}}{{O}_{3}}\]. \[CO(g)+2{{H}_{2}}(g)\underset{C{{r}_{2}}{{O}_{3}}(s)}{\mathop{\xrightarrow{ZnO(g)\,{{250}^{0}}C}}}\,C{{H}_{3}}OH(g)\]
![]()
Note :q Positive catalyst increases the rate by lowering activation energy of reaction. Catalyst changes the mechanism by changing the intermediate i.e. intermediate of low energy is formed. It increases the rate by converting some inactive molecule into active one.
(4) Negative catalysis : There are certain, substance which, when added to the reaction mixture, retard the reaction rate instead of increasing it. These are called negative catalyst or inhibitors and the phenomenon is known as negative catalysis. Some examples are as follows.
(i) The oxidation of sodium sulphite by air is retarded by alcohal. Alcohol acts as a negative catalyst
\[2N{{a}_{2}}S{{O}_{3}}(s)\,{{O}_{2}}(g)\xrightarrow{Alcohol(l)}2N{{a}_{2}}S{{O}_{4}}(s)\]
(ii) The oxidation of chloroform by air is retarded it some alcohol is added to it.
\[2CHC{{l}_{3}}(l)+{{O}_{2}}(g)\xrightarrow{Alcohol(l)}2COC{{l}_{2}}(g)+2HCl(g)\]
(iii) The oxidation of benzaldehyde is retarded if some diphenyl amine is added. It acts as a negative catalyst.
\[2{{C}_{6}}{{H}_{5}}CHO(l)+{{O}_{2}}(g)\underset{amine(l)}{\mathop{\xrightarrow{Diphenyl}}}\,2{{C}_{6}}{{H}_{5}}COOH(l)\]
(iv) Addition of small amount of acetanilide or glycerine slow down the decomposition of hydrogen peroxide.
(v) Tetra ethyl lead (TEL) is added to petrol to retard the ignition of petrol vapours on compression in an internal combustion engine and thus minimise the knocking effect.
(5) Auto-catalysis : In certain reactions, one of the product acts as a catalyst. In the initial stages the reaction is slow but as soon as the products come into existences the reaction rate increases. This type of phenomenon is known as auto-catalysis. Some examples are as follows,
(i) The rate of oxidation of oxalic acid by acidified potassium permanganate increases as the reaction progresses. This acceleration is due to the presence of \[M{{n}^{2+}}\] ions which are formed during reaction. Thus \[M{{n}^{2+}}\]ions act as auto-catalyst. \[5{{H}_{2}}{{C}_{2}}{{O}_{4}}+2KMn{{O}_{4}}+3{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}2MnS{{O}_{4}}+{{K}_{2}}S{{O}_{4}}+10C{{O}_{2}}+8{{H}_{2}}O\]
(i) When nitric acid is poured on copper, the reaction is very slow in the beginning, gradually the reaction becomes faster due to the formation of nitrous acid during the reaction which acts as an auto-catalyst.
(iii) In hydrolysis of ethyl acetate, acetic acid and ethyl alcohol are formed. The reaction is initially very slow but gradually its rate increases. This is due to formation of acetic acid which acts as an auto-catalyst in this reaction.
(6) Induced catalysis : When one reaction influences the rate of other reaction, which does not occur under ordinary conditions, the phenomenon is known as induced catalysis. Some examples are as follows,
(i) Sodium arsenite solution is not oxidised by air. If, however, air is passed through a mixture of the solution of sodium arsenite and sodium sulphite, both of them undergo simultaneous oxidation. The oxidation of sodium sulphite, thus, induces the oxidation of sodium arsenite.
(ii) The reduction of mercuric chloride \[(HgC{{l}_{2}})\]with oxalic acid is very slow, but potassium permanganate is reduced readily with oxalic acid. If, however, oxalic acid is added to a mixture of potassium permanganate and \[HgC{{l}_{2}}\]both are reduced simultaneously. The reduction of potassium permanganate, thus, induces the reduction of mercuric chloride.
(7) Acid-base catalysis : According to the Arrhenius and Ostwald \[{{H}^{+}}\]or\[{{H}^{}}\] ion act as a catalyst.
(i) For example, Hydrolysis of an ester, \[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}(l)+{{H}_{2}}O(l)\underset{O{{H}^{-}}}{\mathop{\xrightarrow{{{H}^{+}}\,or}}}\,C{{H}_{3}}COOH(l)+{{C}_{2}}{{H}_{5}}OH(l)\]
(ii) Inversion of cane sugar, \[\underset{\text{Sugar}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}(l)+{{H}_{2}}O}}\,\xrightarrow{{{H}^{+}}}\underset{\text{Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}(l)}}\,+\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}(l)}}\,\]
(iii) Conversion of acetone into diacetone alcohol,
\[C{{H}_{3}}COC{{H}_{3}}(l)+C{{H}_{3}}COC{{H}_{3}}(l)\xrightarrow{O{{H}^{-}}}C{{H}_{3}}COC{{H}_{2}}.C{{(C{{H}_{3}})}_{2}}OH(l)\]
(iv) Decomposition of nitramide, \[N{{H}_{2}}N{{O}_{2}}(l)\xrightarrow{O{{H}^{-}}}{{N}_{2}}O(g)+{{H}_{2}}O(l)\]
![]()
Note :q All Bronsted acids and bases act as acid base catalysts.
\[\underset{\text{(Unburnt petrol)}}{\mathop{2CO+{{O}_{2}}}}\,\xrightarrow{\text{Catalyst}}2C{{O}_{2}}\]; \[\text{Hydrocarbons}\underset{{{\text{O}}_{\text{2}}}}{\mathop{\xrightarrow{\text{Catalys}t}}}\,C{{O}_{2}}+{{H}_{2}}O\]; \[2NO\xrightarrow{Catalyst}{{N}_{2}}+{{O}_{2}}\]
Characteristics of catalysis.
The following are the characteristics which are common to must of catalytic reactions.
(2) A small quantity of the catalyst is generally sufficient to catalyses almost unlimited reactions
(i) For example, in the decomposition of hydrogen peroxide, one gram of colloidal platinum can catalyses \[{{10}^{8}}\]litres of hydrogen peroxide.
(ii) In the some reaction the rate of the reaction is proportional to the concentration of the catalyst. For example the acid and alkaline hydrolysis of an ester, the rate of reaction is proportional to the concentration of \[{{H}^{+}}\]or \[O{{H}^{-}}\] ions. \[RCOO{{R}^{'}}(l)+{{H}_{2}}O(l)\underset{O{{H}^{-}}}{\mathop{\xrightarrow{{{H}^{+}}or}}}\,RCOOH(l)+{{R}^{'}}OH(l)\]
(iii) In Friedel – craft’s reaction, anhydrous aluminium chloride is required in relatively large amount to the extent of 30% of the mass of benzene, \[{{C}_{6}}{{H}_{6}}+{{C}_{2}}{{H}_{5}}Cl\xrightarrow{AlC{{l}_{3}}}{{C}_{6}}{{H}_{5}}{{C}_{2}}{{H}_{5}}+HCl\]
(iv) In certain heterogeneous reactions, the rate of reaction increases with the increase of area of the catalytic surface.
(3) The catalyst can not initiate the reaction: The function of the catalyst is to alter the speed of the reaction rather than to start it.
(4) The catalyst is generally specific in nature: A substance, which acts as a catalyst for a particular reaction , fails to catalyse the other reaction , different catalysts for the same reactant may for different products.
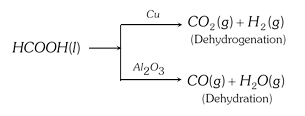
(5) The catalyst cannot change the position of equilibrium : The catalyst catalyse both forward and backward reactions to the same extent in a reversible reaction and thus have no effect on the equilibrium constant.
(6) Catalytic promoters : Substances which themselves are not catalysts, but when mixed in small quantities with the catalysts increase their efficiency are called as promoters or activators.
(i) For example, in Haber’s process for the synthesis of ammonia, traces of molybdenum increases the activity of finely divided iron which acts as a catalyst.
(ii) In the manufacture of methyl alcohol from water gas \[(CO+{{H}_{2}})\], chromic oxide \[(C{{r}_{2}}{{O}_{3}})\] is used as a promoter with the catalyst zinc oxide \[(ZnO)\].
(iii) In the hydrogenation of oils, the activity of the catalyst nickel increases on adding small amount of copper.
(7) Catalytic poisons: Substances which destroy the activity of the catalyst by their presence are known as catalytic poisons.
(i) For example, the presence of traces of arsenious oxide \[(A{{s}_{2}}{{O}_{3}})\] in the reacting gases reduces the activity of platinized asbestos which is used as catalyst in contact process for the manufacture of sulphuric acid.
(ii) The activity of iron catalyst is destroyed by the presence of \[{{H}_{2}}S\]or \[CO\] in the synthesis of ammonia by Haber’s process.
(iii) The platinum catalyst used in the oxidation of hydrogen is poisoned by \[CO\].
. ![]()
Note :q The poisoning of the catalyst is probably due to the preferential adsorption of poison on the surface of the catalyst, thus reducing the space available for the adsorption of reacting molecules.
(8) Change of temperature alters the rate of catalytic reaction as it does for the same reaction in absence of catalyst : By increasing the temperature, there is an increase in the catalytic power of a catalyst but after a certain temperature its power begins to decrease. A catalyst has thus, a particular temperature at which its catalytic activity is maximum. This temperature is termed as optimum temperature.
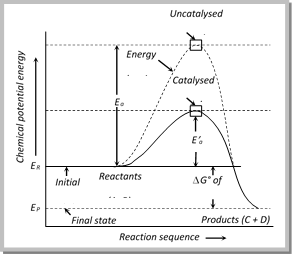
(9) A positive catalyst lowers the activation energy
(i) According to the collision theory, a reaction occurs on account of effective collisions between the reacting molecules.
(ii) For effective collision, it is necessary that the molecules must possess a minimum amount of energy known as activation energy \[\left( {{E}_{a}} \right)\].
(iii) After the collision molecules form an activated complex which dissociate to yield the product molecules.
(iv) The catalyst provides a new pathway involving lower amount of activation energy. Thus,

larger number of effective collisions occur in the presence of a catalyst in comparison to effective collisions at the same temperature in absence of a catalyst. Hence the presence of a catalyst makes the reaction to go faster.
(v) Figure shows that activation energy\[{{E}_{a}}\], in absence of a catalyst is higher than the activation energy\[{{E}_{a}}\], in presence of a catalyst.
(vi) \[{{E}_{R}}\] and \[{{E}_{p}}\] represent the average energies of reactants and products. The difference gives the value of \[\Delta G\], i.e., \[\Delta G={{E}_{R}}-{{E}_{P}}\]
Theories of catalysis.
There are two theories of catalysis which is described as follows.
(1) Intermediate compound theory
(i) This theory was proposed by Clement and Desormes in 1806. According to this theory, the desired reaction is brought about by a path involving the formation of an unstable intermediate compound, followed by its decomposition into the desired end products with the regeneration of the catalyst.
(a) When the intermediate compound is reactive and reacts with the other reactants.
\[AB+X\underset{\text{intermediate}}{\mathop{\to \,\,BX\,\,\,+}}\,A\]
\[BX+C\to CB+X\] …….(i)
(b) When the intermediate is unstable and decomposes to give the final product.
\[A+B+X\underset{\text{intermediate}}{\mathop{\to ABX\to }}\,AB+X\] …….(ii)
Where, A, B and C are the reactant molecules and X is the molecule of the catalyst. The first type of reaction sums up to, \[AB+C\to CB+A\]
While the second to, \[A+B\to AB\] in many cases, the intermediate compounds postulated to be formed are known compounds and often their presence is detected.
(2) Adsorption theory
(i) This theory is applicable to reactions between gases in the presence of a solid catalyst. Some typical examples are as follows.
(ii) The contact process for the oxidation of \[S{{O}_{2}}\] to \[S{{O}_{3}}\] with atmospheric oxygen in the presence of platinum as the catalyst.
(iii) The Haber’s process for the synthesis of ammonia with iron as the catalyst.
(iv) Adsorption results in the loosening of the chemical bonds in the reactant molecules, so that their rupture becomes easier. This is confirmed by the observed lower activation energies for heterogeneous catalytic reactions in the presence of the catalysts as compared to that for the same reaction in the absence of the catalyst.
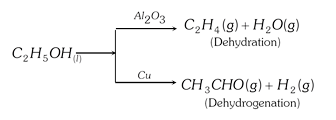
(v) The metals copper and nickel are found particularly suitable for reactions involving hydrogen gas. These metals are known to strongly chemisorb hydrogen gas. Typical example includes the dehydrogenation of ethandol vapours when passed over heated metal at \[{{350}^{o}}C\]. \[C{{H}_{3}}C{{H}_{2}}OH\underset{{{350}^{o}}C}{\mathop{\xrightarrow{Ni}}}\,C{{H}_{3}}CHO+{{H}_{2}}\]
(vi) Aluminium oxide in some physical forms is a good adsorbent for water vapour. It is also a useful catalyst for reactions involving dehydration processes (i.e. processes involving the removal of water from molecules). For example, formation of ethene from ethyl alcohol,
You need to login to perform this action.
You will be redirected in
3 sec
