Colloids, Emulsion, Gel and Their Properties With Application
Category : NEET
Colloids, Emulsion, Gel and Their Properties With Application
Colloidal state.
(1) The foundation of colloidal chemistry was laid down by an English scientist, Thomas Graham, in 1861. The credit for the various advances in this field goes to eminent scientists like Tyndall, Hardy, Zsigmondy, N.R. Dhar, S.S. Bhatnagar and others.
(2) Thomas Graham classified the soluble substances into two categories depending upon the rate of diffusion through animal and vegetable membranes or parchment paper.
(i) Crystalloids : They have higher rate of diffusion and diffused from parchment paper.
Examples : All organic acids, bases and salts and organic compounds such as sugar, urea etc.
(ii) Colloids (Greek word, kolla, meaning glue-like) : They have slower rate of diffusion and can not diffused from parchment paper. Examples : Starch, gelatin, gums, silicic acid and hdemoglobin etc.
(3) The above classification was discarded i.e., the terms colloid does not apply to a particular class of substances but is a state of matter like solid, liquid and gas. Any substance can be brought into colloidal state.
(4) The colloidal state depends on the particle size. If is regarded as intermediate state between true solution and suspension.
detected by any optical means and freely diffuse through membranes. It is a homogenous system.
filter paper. It is a heterogeneous system.
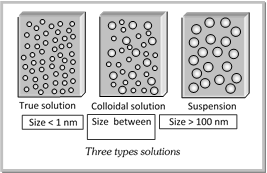
The size of different solutions are sometimes expressed in other units also as given below: Size (diameter) of particles in particles in different units
| True solutions | Colloids | Suspensions | Relation |
| \[<{{10}^{9}}m\] \[<1nm\] \[<10{\AA}\] \[<1000pm\] | \[{{10}^{9}}mto\text{ }{{10}^{7}}m\] 1 nm - 100 nm \[10\text{ }{\AA}1000\text{ }{\AA}\] \[1000pm{{10}^{5}}pm\] | \[>{{10}^{-7}}\]m > 100 nm \[>1000\text{ }{\AA}\] \[>{{10}^{5}}pm\] | \[1nm={{10}^{9}}m\] \[1\text{ }{\AA}={{10}^{10}}m\] \[1pm={{10}^{12}}m\] |
The important distinguishing features of the three types of solutions
| Property | Suspension | Colloid solution | True solution |
| Nature | Heterogeneous | Heterogeneous | Homogeneous |
| Particle size | > 100 nm | 1 nm ? 100 nm | < 1 nm |
| Separation by (i) Ordinary filtration (ii) Ultra- filtration | Possible Possible | Not possible Possible | Not possible Not possible |
| Settling of particles | Settle under gravity | Settle only on centrifugation | Do not settle |
| Appearance | Opaque | Generally transparent | Transparent |
| Tyndall effect | Shows | Shows | Does not show |
| Diffusion of particles | Does not diffuse | Diffuses slowly | Diffuses rapidly |
| Brownian movement | May show | Shows | Negligible |
(5) Roughly speaking the colloidal state is a heterogeneous dispersion of solute particles of size between true solution and suspension. ![]()
Note :q Colloidal particles do not settle down under the force of gravity even an long keeping.
q The surface area of colloidal particle is very large in comparison to suspension.
Phases of colloids and their classification.
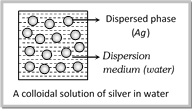
(1) Phases of colloids: We know that a colloidal solution is of heterogeneous nature. It consists of two phases which are as follows
(i) Internal phase or Dispersed phase (Discontinuous phase) : It is the component present in small proportion and is just like a solute in a solution. For example in the colloidal solution of silver in water (silver acts as a dispersed phase)
(ii) External phase or Dispersion medium (continuous phase) : It is generally component present in excess and is just like a solvent in a solution. For example, in the colloidal solution of silver in water. Water act as a dispersion medium.
![]()
Note :q When dispersion medium is a gas, the colloidal system is called aerosol. When it is a liquid system is called solution (hydrosol for water, alcosol for alcohol, benzosol for benzene)
(2) Classification of colloids : The colloids are classified on the basis of the following criteria
(i) Classification based on the physical state of the dispersed phase and dispersion medium Depending upon the physical state of dispersed phase and dispersion medium whether these are solids, liquids or gases, eight types of colloidal systems are possible.
Different types of colloidal systems
| Dispersed phase | Dispersion Medium | Colloidal System | Examples |
| Liquid | Gas | Aerosol of liquids | Fogs, clouds, mists, fine insecticide sprays |
| Solid | Gas | Aerosol of solids | Smoke, volcanic dust, haze |
| Gas | Liquid | Foam or froth | Soap lather. Lemonade froth, foam, whipped cream, soda water |
| Liquid | Liquid | Emulsions | Milk, emulsified oils, medicines |
| Solid | Liquid | Sols | Most paints, starch in water, proteins, gold sol, arsenic sulphide sol, ink |
| Gas | Solid | Solid foam | Pumice stone, styrene rubber, foam rubber |
| Liquid | Solid | Gels | Cheese, butter, boot polish, jelly, curd |
| Solid | Solid | Solid sols (coloured glass) | Ruby glass, some gem stones and alloys |
![]()
Note :q A colloidal system of gas in gas is not possible as gases are completely miscible and always form homogenous true solution.
(ii) Classification based on Nature of interaction between dispersed phase and dispersion medium: Depending upon the nature of interactions between dispersed phase and the dispersion medium, the colloidal solutions can be classified into two types as (a) Lyophilic and (b) Lyophobic sols.
(a) Lyophilic colloids (water loving) : Substances such as proteins, starch and rubber whose molecules are large enough to be close to the lower limit of colloidal range pass readily into colloidal state whenever mixed with suitable solvent. Thus these colloids have strong interaction with the dispersion medium and are called lyophilic colloids (hydrophilic when continuous phase is water)
“or”
“The colloidal solutions in which the particles of the dispersed phase have a great affinity for the dispersion medium, are called lyophilic collodis.”
(b) Lyophobic colloids (water hateing) : Substance such as arsenic sulphide, ferric hydroxide, gold and other metals, which are sparingly soluble and whose molecules are much smaller than the lower colloidal limit, change into colloidal state by aggregation of many individual molecules. “These substances; therefore, do not pass into colloidal state readily and are called lyophobic colloids (hydrophobic when continuous phase is water).”
“or”
“The colloidal solutions in which there is no affinity between particles of the dispersed phase and the dispersion medium are called lyophobic colloids.”
Distinction between Lyophilic and Lyophobic Sols
| Property | Lyophilic (suspensoid) | Lyophobic Sols (Emulsoid ) |
| Surface tension | Lower than that of the medium | Same as that of the medium |
| Viscosity | Much higher than that of the medium | Same as that of the medium |
| Reversibility | Reversible | Irreversible |
| Stability | More stable | Less stable |
| Visibility | Particles can?t be detected even under ultramicroscope | Particles can be detected under ultramicroscope. |
| Migration | Particles may migrate in either direction or do not migrate in an electric field because do not carry any charge. | Particles migrate either towards cathode or anode in an electric field because they carry charge. |
| Action of electrolyte | Addition of smaller quantity of electrolyte has little effect | Coagulation takes place |
| Hydration | Extensive hydration takes place | No hydration |
| Examples | Gum, gelatin, starch, proteins, rubber etc. | Metals like Ag and Au, hydroxides like \[Al{{(OH)}_{3}}\], \[Fe{{(OH)}_{3}}\]metal sulphides like \[A{{S}_{2}}{{S}_{3}}\] etc. |
(iii) Classification based on types of particle of dispersed phase: Depending upon the type of the particles of the dispersed phase, the colloids are classified as follows.
(a) Multimolecular colloids
(b) Macromolecular colloids
(c) Associated colloids
Micelles
\[\underset{\text{Sodium stearate}}{\mathop{{{C}_{17}}{{H}_{35}}COONa}}\,\]\[\underset{\text{Stearate ion}}{\mathop{{{C}_{17}}{{H}_{35}}CO{{O}^{-}}}}\,+N{{a}^{+}}\]
The stearate ions associate to form ionic micelles of colloidal size.
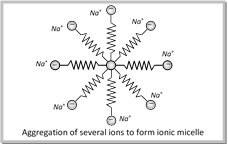
Some other examples of micelles are sodium palmitate \[({{C}_{15}}{{H}_{31}}COONa)\], Sodium lauryl sulphate \[[C{{H}_{3}}{{(C{{H}_{2}})}_{11}}S{{O}_{3}}{{O}^{-}}N{{a}^{+}}]\], Cetyl trimethyl ammonium bromide \[C{{H}_{3}}{{(C{{H}_{2}})}_{15}}{{(C{{H}_{2}})}_{3}}{{N}^{+}}B{{r}^{-}}\] etc.
![]()
Note :q Polydisperse and Monodisperse colloids : In multimolecular colloids, the colloidal particles consist of aggregates of atoms or small molecules with diameters less than \[{{10}^{-9}}\]m of 1 nm Colloidal solutions in which colloidal particles are of different sizes are called polydisperse colloids. For example, a gold sol may contain particles of various sizes having several atoms of gold. The colloidal solutions in which all the colloidal particles are more or less of identical size are monodisperse colloids.
Emulsion.
“The colloidal systems in which fine droplets of one liquid are dispersed in another liquid are called emulsions the two liquids otherwise being mutually immiscible.” or
“Emulsion are the colloidal solutions in which both the dispersed phase and the dispersion medium are liquids.”
A good example of an emulsion is milk in which fat globules are dispersed in water. The size of the emulsified globules is generally of the order of \[{{10}^{-6}}\]m. Emulsion resemble lyophobic sols in some properties.
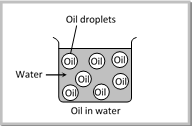
(1) Types of Emulsion : Depending upon the nature of the dispersed phase, the emulsions are classified as;
(i) Oil-in-water emulsions (O/W) : The emulsion in which oil is present as the dispersed phase and water as the dispersion medium (continuous phase) is called an oil-in-water emulsion. Milk is an example of the oil-in-water type of emulsion. In milk liquid fat globules are dispersed in water. Other examples are, vanishing cream etc.
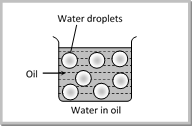
(ii) Water-in-oil emulsion (W/O) : The emulsion in which water forms the dispersed phase, and the oil acts as the dispersion medium is called a water-in-oil emulsion. These emulsion are also termed oil emulsions. Butter and cold cream are typical examples of this types of emulsions. Other examples are cod liver oil etc.
![]()
Note :q The emulsion can be inter converted by simply changing the ratio of the dispersed phase and dispersion medium. For example, an oil-in-water emulsion can be converted to water in oil emulsion by simply adding excess of oil in the first case.
(2) Preparation of Emulsions
(i) Emulsions are generally prepared by vigorously agitating a mixture of the relevant oil and water by using either a high speed mixer or by using ultrasonic vibrators.
(ii) The emulsions obtained by simple mechanical stirring are unstable. The two components (oil and water) tend to separate out.
(iii) To obtain a stable emulsion, a suitable stabilizing substance is generally added.
(iv) The stabilizing substance is called emulsifier of emulsifying agent. The emulsifier is added along with the oil and water in the beginning. For Examples : substances which can act as emulsifiers are soaps, detergents, long chain sulphonic acid, lyophilic colloids like gelatin, albumin, casein etc.
(3) Nature of emulsifier : Different emulsifiers may act differently in the case of a particular emulsion.
For example,
(4) Identification of emulsions : Several methods are available to find out whether an emulsion is of the oil-in-water type or of the water-in-oil type emulsion. An emulsion can be identified as follows.
(i) Dilute test : Add water to the emulsion. If the emulsion can be diluted with water this means that water acts as the dispersion medium and it is an example of oil-in-water emulsion. In case it is not diluted, then oil acts as dispersion medium and it is an example of water-in-oil emulsion.
(ii) Dye test : An oil soluble suitable dye is shaken with the emulsion. If colour is noticed on looking at a drop of the emulsion, it is oil-in-water type emulsion. In case the entire background is coloured, it is an example of water-in-oil type.
(iii) Conductivity test : Add small amount of an electrolyte (e.g. KCl) to the emulsion. If this makes the emulsion electrically conducting , then water is the dispersion medium. If water is not the dispersed phase.
(5) Properties of emulsion
(i) Emulsions show all the characteristic properties of colloidal solution such as Brownian movement, Tyndall effect, electrophoresis etc.
(ii) These are coagulated by the addition of electrolytes containing polyvalent metal ions indicating the negative charge on the globules.
(iii) The size of the dispersed particles in emulsions in larger than those in the sols. It ranges from 1000 Å to 10,000 Å. However, the size is smaller than the particles in suspensioins.
(iv) Emulsions can be converted into two separate liquids by heating, centrifuging, freezing etc. This process is also known as demulsification.
(6) Applications of emulsions
(i) Concentration of ores in metallurgy
(ii) In medicine (Emulsion water-in-oil type)
(iii) Cleansing action of soaps.
(iv) Milk, which is an important constituent of our diet an emulsion of fat in water.
(v) Digestion of fats in intestine is through emulsification.
Gels.
(1) “A gel is a colloidal system in which a liquid is dispersed in a solid.”
(2) The lyophilic sols may be coagulated to give a semisolid jelly like mass, which encloses all the liquid present in the sol. The process of gel formation is called gelation and the colloidal system formed called gel.
(3) Some gels are known to liquify on shaking and reset on being allowed to stand. This reversible sol-gel transformation is called thixotropy.
(4) The common examples of gel are gum arabic, gelatin, processed cheese, silicic acid, ferric hydroxide etc.
(5) Gels may shrink by loosing some liquid help them. This is known as synereises or weeping.
(6) Gels may be classified into two types
(i) Elastic gels : These are the gels which possess the property of elasticity. They readily change their shape on applying force and return to original shape when the applied force is removed. Common examples are gelatin, agar-agar, starch etc.
(ii) Non-elastic gels : These are the gels which are rigid and do not have the property of elasticity. For example, silica gel.
Application of colloids.
(1) Purification of water by alum (coagulation): Alum which yield \[A{{l}^{3+}}\]ions, is added to water to coagulate the negatively charged clay particles.
(2) In rubber and tanning industry (coagulation and mutual coagulation) : Several industrial processes such as rubber plating, chrome tanning, dyeing, lubrication etc are of colloidal nature
(i) In rubber platting, the negatively charged particles of rubber (latex) are made to deposit on the wires or handle of various tools by means of electrophoresis. The article on which rubber is to be deposited is made anode.
(ii) In tanning the positively charged colloidal particles of hides and leather are coagulated by impregnating, them in negatively charged tanning materials (present in the barks of trees). Among the tanning agent chromium salts are most commonly used for the coagulation of the hide material and the process is called chrome tanning.
(3) Artificial rains : It is possible to cause artificial rain by throwing the electrified sand or silver iodide from an aeroplane and thus coagulating the mist hanging in air.
(4) Smoke precipitation (Coagulation) : Smoke is a negative sol consisting of carbon particles dispersed in air. Thus, these particles are removed by passing through a chamber provided with highly positively charged metallic knob.
(5) Formation of deltas (coagulation) : River water consists of negatively charged clay particles of colloidal dimension. When the river falls into the sea, the clay particles are coagulated by the positive \[N{{a}^{+}},\,{{K}^{+}},\,M{{g}^{2+}}\] ions etc. present in sea water and new lands called deltas are formed.
(6) Blood consists of negatively charged colloidal particles (albuminoid substance). The colloidal nature of blood explains why bleeding stops by applying a ferric chloride solution to the wound. Actually ferric chloride solution causes coagulation of blood to form a clot which stops further bleeding.
(7) Colloidal medicine : Argyrol and protargyrol are colloidal solution of silver and are used as eye lotions colloidal sulphur is used as disinfectant colloidal gold, calcium and iron are used as tonics.
(8) Photographic plates : These are thin glass plates coated with gelatin containing a fine suspension of silver bromide. The particles of silver bromide are colloidal in nature.
![]()
Note : q Isoelectric point of the colloid : The hydrogen ion concentration at which the colloidal particles are neither positively charged nor negatively charged (neutral) is known as isoelectric point of the colloids. At this point, the lyophilic colloids are expected to have minimum stability because at this point particles have no charge or equal quantum of positively and negatively charge. For example,
isoelectric point of gelatin is 4.7 (at pH 4.7 gelatin has no electrophoretic motion; at pH<4.7, gelatin moves towards anode)
(i) Scanning Electron Microscope (SEM), (ii) Transmission Electron Microscope (TEM)
A modified form of the above methods has also been developed. It is called Scanning Transmission Electron Microscope (STEM). All these techniques are superior to the light microscope because they have greater resolving power.
Bencroft rule : The phase in which the emulsifier is more soluble becomes outer phase of the emulsion.
You need to login to perform this action.
You will be redirected in
3 sec
