Atomic number, Mass number Atomic species
Category : NEET
Atomic Number, Mass Number and Atomic Species
(1) Atomic number or Nuclear charge
(i) The number of protons present in the nucleus of the atom is called atomic number (Z).
(ii) It was determined by Moseley as,
![]()
where, \[\nu =X-\]rays frequency
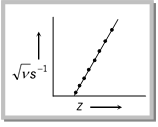
Z= atomic number of the metal
\[a\And b\] are constant.
(iii) Atomic number = Number of positive charge on nucleus = Number of protons in nucleus = Number of electrons in nutral atom.
(iv) Two different elements can never have identical atomic number.
(2) Mass number
(i) The sum of proton and neutrons present in the nucleus is called mass number.
Mass number (A) = Number of protons + Number of neutrons or Atomic number (Z)
or Number of neutrons = A – Z .
(ii) Since mass of a proton or a neutron is not a whole number (on atomic weight scale), weight is not necessarily a whole number.
(iii) The atom of an element X having mass number (A) and atomic number (Z) may be represented by a symbol,

![]()
Note : q A part of an atom up to penultimate shell is a kernel or atomic core.
q Negative ion is formed by gaining electrons and positive ion by the loss of electrons.
q Number of lost or gained electrons in positive or negative ion =Number of protons \[\pm \]charge on ion.
(3) Different Types of Atomic Species
|
Atomic species |
Similarities |
Differences |
Examples |
|
Isotopes (Soddy) |
(i) Atomic No. (Z) (ii) No. of protons (iii) No. of electrons (iv) Electronic configuration (v)Chemical properties (vi) Position in the periodic table |
(i) Mass No. (A) (ii) No. of neutrons (iii) Physical properties |
(i) \[_{1}^{1}H,\,_{1}^{2}H,\,_{1}^{3}H\] (ii) \[_{8}^{16}O,\,_{8}^{17}O,\,_{8}^{18}O\] (iii) \[_{17}^{35}Cl,\,_{17}^{37}Cl\]
|
|
Isobars |
(i) Mass No. (A) (ii) No. of nucleons |
(i) Atomic No. (Z) (ii) No. of protons, electrons and neutrons (iii)Electronic configuration (iv) Chemical properties (v) Position in the perodic table. |
(i) \[_{18}^{40}Ar,\,_{19}^{40}K,\,_{20}^{40}Ca\] (ii) \[_{52}^{130}Te,\,_{54}^{130}Xe,\,_{56}^{130}Ba\] |
|
Isotones |
No. of neutrons |
(i) Atomic No. (ii) Mass No., protons and electrons. (iii) Electronic configuration (iv) Physical and chemical properties (v) Position in the periodic table. |
(i) \[_{14}^{30}Si,\,_{15}^{31}P,\,_{16}^{32}S\] (ii) \[_{19}^{39}K,\,_{20}^{40}Ca\] (iii) \[_{1}^{3}H,\,_{2}^{4}He\] (iv) \[_{6}^{13}C,\,_{7}^{14}N\] |
|
Isodiaphers |
Isotopic No. (N ? Z) or (A ? 2Z) |
(i) At No., mass No., electrons, protons, neutrons. (ii) Physical and chemical properties. |
(i)\[_{92}{{U}^{235}},{{\,}_{90}}T{{h}^{231}}\] (ii) \[_{19}{{K}^{39}},{{\,}_{9}}{{F}^{19}}\] (iii) \[_{29}C{{u}^{65}},{{\,}_{24}}C{{r}^{55}}\] |
|
Isoelectronic species |
(i) No. of electrons (ii) Electronic configuration |
At. No., mass No. |
(i) \[{{N}_{2}}O,\,C{{O}_{2}},\,CN{{O}^{-}}(22{{e}^{-}})\] (ii) \[CO,\,C{{N}^{-}},\,{{N}_{2}}(14{{e}^{-}})\] (iii) \[{{H}^{-}},\,He,\,L{{i}^{+}},\,B{{e}^{2+}}(2{{e}^{-}})\] (iv) \[{{P}^{3-}},\,{{S}^{2-}},\,C{{l}^{-}},\,Ar,\,{{K}^{+}}and\,C{{a}^{2+}}(18{{e}^{-}})\]
|
|
Isosters |
(i) No. of atoms (ii) No. of electrons (iii) Same physical and chemical properties. |
|
(i) \[{{N}_{2}}\] and \[CO\] (ii) \[C{{O}_{2}}\] and \[{{N}_{2}}O\] (iii) \[HCl\] and \[{{F}_{2}}\] (iv) \[CaO\] and \[MgS\] (v) \[{{C}_{6}}{{H}_{6}}\] and \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\] |
![]()
Note : q In all the elements, tin has maximum number of stable isotopes (ten).
q Average atomic weight/ The average isotopic weight
\[=\frac{\text{ }\!\!%\!\!\text{ of 1st isotope }\times \text{ relative mass of 1st isotope }+\text{ }\!\!%\!\!\text{ of 2nd isotope }\times \text{ relative mass of 2nd isotope}}{\text{100}}\]
Example : 4 The characteristics X- ray wavelength for the lines of the \[{{k}_{\alpha }}\]series in elements X and Y are 9.87Å and 2.29Å respectively. If Moseley’s equation\[\sqrt{\nu }=4.9\times {{10}^{7}}(Z-0.75)\] is followed, the atomic numbers of X and Y are
(a) 12, 24 (b) 10, 12 (c) 6, 12 (d) 8, 10
Solution : (a) \[\nu =\frac{c}{\lambda }\]
\[\sqrt{{{\nu }_{x}}}=\sqrt{\frac{3\times {{10}^{8}}}{9.87\times {{10}^{-10}}}}=5.5132\times {{10}^{8}}\]
\[\sqrt{{{\nu }_{y}}}=\sqrt{\frac{3\times {{10}^{8}}}{2.29\times {{10}^{-10}}}}=11.4457\times {{10}^{8}}\]
using Moseley’s equation we get
\[\therefore \,\,\,\,5.5132\times {{10}^{8}}=4.9\times {{10}^{7}}({{Z}_{x}}-0.75)\] …..(i)
and \[11.4457\times {{10}^{8}}=4.90\times {{10}^{7}}({{Z}_{y}}-0.75)\] ….. (ii)
On solving equation (i) and (ii) \[{{Z}_{x}}=12,\,\,{{Z}_{y}}=24.\]
Example : 5 If the straight line is at an angle 45° with intercept, 1 on \[\sqrt{\nu }-\text{axis,}\] calculate frequency \[\nu \]when atomic number Z is 50.
(a) 2000\[{{s}^{-1}}\] (b) 2010\[{{s}^{-1}}\] (c) 2401\[{{s}^{-1}}\] (d) None
Solution : (c) \[\sqrt{\nu }=\tan \,45{}^\circ =1=a\]
ab=1
\[\therefore \,\,\sqrt{\nu }=50-1=49\]
\[\nu =2401\,{{s}^{-1}}.\]

Example : 6 What is atomic number Z when \[\nu =2500\,{{s}^{-1}}\]?
(a) 50 (b) 40 (c) 51 (d) 53
Solution : (c) \[\sqrt{\nu }=\sqrt{2500}=Z-1,\,\ \ \ Z=51.\]
Example : 7 Atomic weight of \[Ne\] is 20.2. \[Ne\] is a mixutre of \[N{{e}^{20}}\] and \[N{{e}^{22}}\]. Relative abundance of heavier isotope is
(a) 90 (b) 20 (c) 40 (d) 10
Solution:(d) Average atomic weight/ The average isotopic weight
\[=\frac{\text{ }\!\!%\!\!\text{ of 1st isotope }\times \text{ relative mass of 1st isotope }+\text{ }\!\!%\!\!\text{ of 2nd isotope }\times \text{ relative mass of 2nd isotope}}{\text{100}}\]
\[\therefore \ \ 20.2=\frac{a\times 20+(100-a)\times 22}{100}\]; \[\therefore \ \ a=90\]; per cent of heavier isotope \[=100-90=10\]
Example : 8 The relative abundance of two isotopes of atomic weight 85 and 87 is 75% and 25% respectively. The average atomic weight of element is
(a) 75.5 (b) 85.5 (c) 87.5 (d) 86.0
Solution:(b) Average atomic weight/ The average isotopic weight
\[=\frac{\text{ }\!\!%\!\!\text{ of 1st isotope }\times \text{ relative mass of 1st isotope }+\text{ }\!\!%\!\!\text{ of 2nd isotope }\times \text{ relative mass of 2nd isotope}}{\text{100}}\]
\[=\frac{85\times 75+87\times 25}{100}=85.5\]
Example : 9 Nitrogen atom has an atomic number of 7 and oxygen has an atomic number of 8. The total number of electrons in a nitrate ion is
(a) 30 (b) 35 (c) 32 (d) None
Solution : (c) Number of electrons in an element = Its atomic number
So number of electrons in N=7 and number of electrons in O=8.
Formula of nitrate ion is \[NO_{3}^{-}\]
So, in it number of electrons
\[=1\times \] number of electrons of nitrogen \[+3\times \] number of electrons of oxygen +1 (due to negative charge)
\[=1\times 7+3\times 8+1=32\]
Example :10 An atom of an element contains 11 electrons. Its nucleus has 13 neutrons. Find out the atomic number and approximate atomic weight.
(a) 11, 25 (b) 12, 34 (c) 10, 25 (d) 11, 24
Solution : (d) Number of electrons =11
\[\therefore \] Number of protons = Number of electron =11
Number of neutrons = 13
Atomic number of element = Number of proton = Number of electrons =11
Further, Atomic weight = Number of protons + Number of neutrons =11 + 13=24
Example : 11 How many protons, neutrons and electrons are present in \[(a)\,\,_{15}^{31}P\]\[(b)\,\,_{18}^{40}Ar\]\[(c)\,\,_{47}^{108}Ag\]?
Solution : The atomic number subscript gives the number of positive nuclear charges or protons. The neutral atom contains an equal number of negative electrons. The remainder of the mass is supplied by neutrons.
|
Atom |
Protons |
Electrons |
Neutrons |
|
\[_{15}^{31}P\] |
15 |
15 |
31 ? 15=16 |
|
\[_{18}^{40}Ar\] |
18 |
18 |
40 ? 18=22 |
|
\[_{47}^{108}Ag\] |
47 |
47 |
108 ? 47=61 |
Example :12 State the number of protons, neutrons and electrons in \[{{C}^{12}}\]and \[{{C}^{14}}.\]
Solution : The atomic number of \[{{C}^{12}}\]is 6. So in it number of electrons = 6
Number of protons =6; Number of neutrons =12 – 6=6
The atomic number of \[{{C}^{14}}\] is 6. So in it number of electrons = 6
Number of protons = 6; Number of neutrons =14 – 6=8
Example :13 Predict the number of electrons, protons and neutrons in the two isotopes of magnesium with atomic number 12 and atomic weights 24 and 26.
Solution : Isotope of the atomic weight 24, i.e. \[_{12}M{{g}^{24}}.\]We know that
Number of protons = Number of electrons =12
Further, Number of neutrons = Atomic weight – Atomic number =24 – 12 =12
Similarly, In isotope of the atomic weight 26, i.e. \[_{12}M{{g}^{26}}\]
Number of protons = Number of electrons =12
Number of neutrons = 26 – 12 = 14
Electromagnetic Radiations.
(1) Light and other forms of radiant energy propagate without any medium in the space in the form of waves are known as electromagnetic radiations. These waves can be produced by a charged body moving in a magnetic field or a magnet in a electric field. e.g. \[\alpha -\]rays, \[\gamma -\]rays, cosmic rays, ordinary light rays etc.
(2) Characteristics : (i) All electromagnetic radiations travel with the velocity of light. (ii) These consist of electric and magnetic fields components that oscillate in directions perpendicular to each other and perpendicular to the direction in which the wave is travelling.
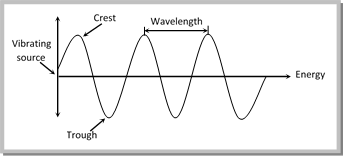
(3) A wave is always characterized by the following five characteristics:
(i) Wavelength : The distance between two nearest crests or nearest troughs is called the wavelength. It is denoted by \[\lambda \](lambda) and is measured is terms of centimeter (cm), angstrom (Å), micron(\[\mu \]) or nanometre (nm).
\[1{\AA}={{10}^{-8}}\,cm={{10}^{-10}}m\]
\[1\mu ={{10}^{-4}}cm={{10}^{-6}}m\]
\[1nm={{10}^{-7}}cm={{10}^{-9}}m\]
\[1cm={{10}^{8}}{\AA}={{10}^{4}}\mu ={{10}^{7}}nm\]
(ii) Frequency : It is defined as the number of waves which pass through a point in one second. It is denoted by the symbol \[\nu \](nu) and is expressed in terms of cycles (or waves) per second (cps) or hertz (Hz).
\[\lambda \nu =\]distance travelled in one second = velocity =c
\[\nu =\frac{c}{\lambda }\]
(iii) Velocity : It is defined as the distance covered in one second by the wave. It is denoted by the letter ‘c’. All electromagnetic waves travel with the same velocity, i.e., \[3\times {{10}^{10}}cm/\sec .\]
\[c=\lambda \nu =3\times {{10}^{10}}\ cm/\sec \]
Thus, a wave of higher frequency has a shorter wavelength while a wave of lower frequency has a longer wavelength.
(iv) Wave number : This is the reciprocal of wavelength, i.e., the number of wavelengths per centimetre. It is denoted by the symbol \[\bar{\nu }\](nu bar). It is expressed in\[c{{m}^{-1}}\,\text{or}\,{{m}^{-1}}\].
\[\bar{\nu }=\frac{1}{\lambda }\]
(v) Amplitude : It is defined as the height of the crest or depth of the trough of a wave. It is denoted by the letter ‘A’. It determines the intensity of the radiation.
The arrangement of various types of electromagnetic radiations in the order of their increasing or decreasing wavelengths or frequencies is known as electromagnetic spectrum.
|
Name |
Wavelength (Å) |
Frequency (Hz) |
Source |
|
Radio wave |
\[3\times {{10}^{14}}-3\times {{10}^{7}}\] |
\[1\times {{10}^{5}}-1\times {{10}^{9}}\] |
Alternating current of high frequency |
|
Microwave |
\[3\times {{10}^{7}}-6\times {{10}^{6}}\] |
\[1\times {{10}^{9}}-5\times {{10}^{11}}\] |
Klystron tube |
|
Infrared (IR) |
\[6\times {{10}^{6}}-7600\] |
\[5\times {{10}^{11}}-3.95\times {{10}^{16}}\] |
Incandescent objects |
|
Visible |
\[7600-3800\] |
\[3.95\times {{10}^{16}}-7.9\times {{10}^{14}}\] |
Electric bulbs, sun rays |
|
Ultraviolet (UV) |
\[3800-150\] |
\[\text{7}\text{.9}\times \text{1}{{\text{0}}^{\text{14}}}-2\times {{10}^{16}}\] |
Sun rays, arc lamps with mercury vapours |
|
X-Rays |
\[150-0.1\] |
\[2\times {{10}^{16}}-3\times {{10}^{19}}\] |
Cathode rays striking metal plate |
|
\[\gamma -\]Rays |
\[0.1-0.01\] |
\[\text{3}\times \text{1}{{\text{0}}^{\text{19}}}-3\times {{10}^{20}}\] |
Secondary effect of radioactive decay |
|
Cosmic Rays |
0.01- zero |
\[3\times {{10}^{20}}-\]infinity |
Outer space. |
You need to login to perform this action.
You will be redirected in
3 sec
