Real and Ideal gases
Category : JEE Main & Advanced
(1) Gases which obey gas laws or ideal gas equation \[(PV=nRT)\] at all temperatures and pressures are called ideal or perfect gases. Almost all gases deviate from the ideal behaviour i.e., no gas is perfect and the concept of perfect gas is only theoretical.
(2) Gases tend to show ideal behaviour more and more as the temperature rises above the boiling point of their liquefied forms and the pressure is lowered. Such gases are known as real or non ideal gases. Thus, a “real gas is that which obeys the gas laws under low pressure or high temperature”.
(3) The deviations can be displayed, by plotting the P-V isotherms of real gas and ideal gas.
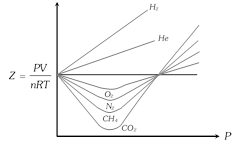
(4) It is difficult to determine quantitatively the deviation of a real gas from ideal gas behaviour from the P-V isotherm curve as shown above. Compressibility factor Z defined by the equation,
\[PV=ZnRT\] or \[Z=PV/nRT=P{{V}_{m}}/RT\]
is more suitable for a quantitative description of the deviation from ideal gas behaviour.
(5) Greater is the departure of Z from unity, more is the deviation from ideal behaviour. Thus, when
(i) \[Z=1\], the gas is ideal at all temperatures and pressures. In case of \[{{N}_{2}}\], the value of Z is close to 1 at \[{{50}^{o}}C\]. This temperature at which a real gas exhibits ideal behaviour, for considerable range of pressure, is known as Boyle's temperature or Boyle's point \[({{T}_{B}})\].
(ii) \[Z>1\], the gas is less compressible than expected from ideal behaviour and shows positive deviation, usual at high P i.e. \[PV>RT\].
(iii) \[Z<1\], the gas is more compressible than expected from ideal behaviour and shows negative deviation, usually at low P i.e. \[PV<RT\].
(iv) \[Z>1\] for \[{{H}_{2}}\] and He at all pressure i.e., always shows positive deviation.
(v) The most easily liquefiable and highly soluble gases \[(N{{H}_{3}},\,S{{O}_{2}})\] show larger deviations from ideal behaviour i.e. \[Z<<1\].
(vi) Some gases like \[C{{O}_{2}}\] show both negative and positive deviation.
(6) Causes of deviations of real gases from ideal behaviour : The ideal gas laws can be derived from the kinetic theory of gases which is based on the following two important assumptions,
(i) The volume occupied by the molecules is negligible in comparison to the total volume of gas.
(ii) The molecules exert no forces of attraction upon one another. It is because neither of these assumptions can be regarded as applicable to real gases that the latter show departure from the ideal behaviour.
You need to login to perform this action.
You will be redirected in
3 sec
