Classification and Nomenclature of Organic Compounds
Category : NEET
Classification and Nomenclature of Organic Compounds
Classification of organic compounds
Organic compounds have been classified on the basis of carbon skeleton (structure) or functional groups or the concept of homology.
(1) Classification based on structure
(i) Acyclic or open-chain compounds: Organic compounds in which all the carbon atoms are linked to one another to form open chains (straight or branched) are called acyclic or open chain compounds. These may be either saturated or unsaturated. For example,
\[\underset{\text{Butane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,\]
\[\underset{\text{Isobutane}}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C{{H}_{3}}-C-C\equiv CH}}\,}}\,}}\,\]
These compounds are also called as aliphatic compounds.
(ii) Cyclic or closed-chain compounds: Cyclic compounds contain at least one ring or closed chain of atoms. The compounds with only one ring of atoms in the molecule are known as monocyclic but those with more than one ring of atoms are termed as polycyclic. These are further divided into two subgroups.
(a) Homocyclic or carbocyclic: These are the compounds having a ring or rings of carbon atoms only in the molecule. The carbocyclic or homocyclic compounds may again be divided into two types:
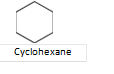
Alicyclic compounds: These are the compounds which contain rings of three or more carbon atoms. These resemble with aliphatic compounds than aromatic compounds in many respects. That is why these are named alicyclic, i.e., aliphatic cyclic. These are also termed as polymethylenes. Some of the examples are,


Aromatic compounds: These compounds consist of at least one benzene ring, i.e., a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and hence, named as aromatic (Greek word aroma meaning sweet smell).
These are also called benzenoid aromatics.

![]()
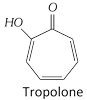
Note:q Non-benzenoid aromatics: There are aromatic compounds, which have structural units different from benzenoid type and are known as Non-benzenoid aromatics e.g. Tropolone, azulene etc.

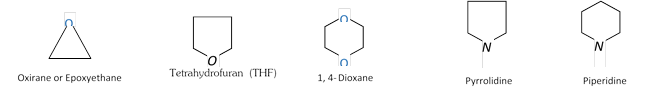
(b) Heterocyclic compounds: Cyclic compounds containing one or more hetero atoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types:
Alicyclic heterocyclic compounds: Heterocyclic compounds which resemble aliphatic compounds in their properties are called Alicyclic heterocyclic compounds. For example,
Aromatic heterocyclic compounds: Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called Aromatic heterocyclic compounds. For example,

(2) Classification based on functional groups: A functional group is an atom or group of atoms in a molecule that gives the molecule its characteristic chemical properties. Double and triple bonds are also considered as functional groups.
All compounds with the same functional group belong to the same class. Various classes of compounds having some of the common functional groups are listed in the table.
| Class | Functional group | Class | Functional group |
| Olefins/Alkenes | Acid halides | \[\overset{O\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,}{\mathop{-C-X}}\,}}\,\] (Acylhalide) | |
| Acetylenes/Alkynes | \[-C\equiv C-\] | Amides | \[\overset{O\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-C-N{{H}_{2}}}}\,}}\,\](Amide) |
| Alkyl Halides | \[-F,\,-Cl,\,-Br,\,-I\,\text{(Halo)}\] | Acid anhydrides | \[\overset{O\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,}{\mathop{-C-O}}\,}}\,\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,\] (Anhydride) |
| Alcohols | -OH (Hydroxy) | Esters | \[\overset{O\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,}{\mathop{-C-O}}\,}}\,\underset{|}{\overset{|}{\mathop{-C-}}}\,\] (Ester) |
| Ethers | \[\underset{|\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{-C-O}}}\,\underset{|}{\overset{|}{\mathop{-C-}}}\,\,\text{(Alkoxy)}\] | Cyanides/Nitriles | \[-C\equiv N\] (Cyano) |
| \[\underset{O\,\,\,\,\,}{\mathop{\underset{||\,\,\,\,\,\,\,}{\mathop{-C-H}}\,}}\,\,\](Aldehydic) | Isocyanides | ? N C (Isocyano) | |
| Ketones | \[\overset{O}{\mathop{\overset{||}{\mathop{-C-}}\,}}\,\] (ketonic) | Nitro compounds | (Nitro) ¯ |
| Carboxylic acid | \[\overset{O\,\,\,\,\,\,\,\,}{\mathop{\overset{||\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-C-OH}}\,}}\,\] (Carboxyl) | Amines | |
(3) Homologous series: A homologous series can be defined as a group of compounds in which the various members have similar structural features and similar chemical properties and the successive members differ in their molecular formula by \[C{{H}_{2}}\].
Characteristics of homologous series
(i) All the members of a series can be represented by the general formula. For example, the members of the alcohol family are represented by the formula \[{{C}_{n}}{{H}_{2n+1}}OH\] where n may have values 1, 2, 3..... etc.
(ii) Two successive members differ in their formula by \[-C{{H}_{2}}\] group or by 14 atomic mass units \[(12+2\times 1)\].
(iii) Different members in a family have common functional group e.g., the members of the alcohol family have \[-OH\] group as the functional group.
(iv) The members in any particular family have almost identical chemical properties and their physical properties such as melting point, boiling point, density, solubility etc., show a proper gradation with the increase in the molecular mass.
(v) The members present in a particular series can be prepared almost by similar methods known as the general methods of preparation.
(4) Saturated and unsaturated compounds : If, in an organic compound containing two or more carbon atoms, there are only single bonds between carbon atoms, then the compound is said to be saturated, e.g. ethane, n-propyl alcohol, acetaldehyde etc.
\[\underset{\,\,\,\,\,\,\,\,\,H}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,H}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{H-C}}}\,}}\,}}\,\underset{H\,\,\,\,\,}{\mathop{\overset{H\,\,\,\,}{\mathop{\underset{\,\,|\,\,\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{-C-H}}}\,;}}\,}}\,\] \[\underset{\text{n-propyl alcohol}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,H}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,H}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{H-C}}}\,}}\,}}\,-\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{H\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{H\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C-O-H;}}}\,}}\,}}\,}}\,\]
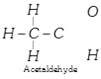
On the other hand, if the compound contains at least one pair of adjacent carbon atoms linked by a multiple bond, then that compound is said to be unsaturated, e.g, ethylene, acetylene, vinyl alcohol, acraldehyde etc.
 ;
; ![]() ;
; 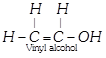
![]()
Note: The double bond between carbon and oxygen atoms is not a sign of unsaturation as in acetaldehyde or acetone.
You need to login to perform this action.
You will be redirected in
3 sec
