Aromatic Nitro Compounds
Category : JEE Main & Advanced
Aromatic nitro compounds are the derivatives of aromatic hydrocarbons in which one or more hydrogen atom (s) of the benzene nucleus has been replaced by nitro \[(\text{ }N{{O}_{2}})\] group.
(1) Preparation
(i) Nitration (Direct method) : The number of \[\text{ }N{{O}_{2}}\] groups introduced in benzene nucleus depends upon the nature and concentration of the nitrating agent, temperature of nitration and nature of the compound to be nitrated.
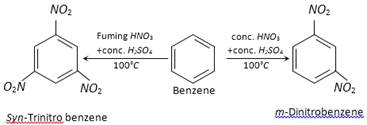
(a) The nature of the nitrating agent : For example,
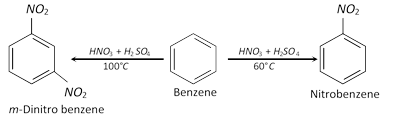
(b) Temperature of nitration : For example,
(c) Nature of the compound to be nitrated : Presence of electron-releasing group like \[OH,N{{H}_{2}},~C{{H}_{3}},OR,\] etc., in the nucleus facilitates nitration. Thus aromatic compounds bearing these groups (i.e. phenol, aniline, toluene, etc.) can be nitrated readily as compared to benzene. Thus benzene is not affected by dilute HNO3 while phenol, aniline and toluene forms the corresponding ortho- and para-nitro compounds.


On the other hand, nitration of aromatic compounds having electron withdrawing groups like \[N{{O}_{2}},S{{O}_{3}}H\] requires powerful nitrating agent (like fuming \[HN{{O}_{3}}+\] conc. \[{{H}_{2}}S{{O}_{4}}\]) and a high temperature.
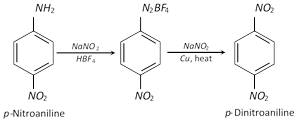
(ii) Indirect method : The aromatic nitro compounds which can not be prepared by direct method may be prepared from the corresponding amino compound.
(2) Physical properties
(i) Aromatic nitro compounds are insoluble in water but soluble in organic solvents.
(ii) They are either pale yellow liquids or solids having distinct smells. For example, nitro benzene (oil of Mirabane) is a pale yellow liquid having a smell of bitter almonds.
(3) Chemical properties
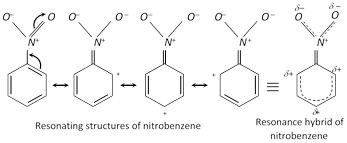
(i) Resonance in nitrobenzene imparts a partial double bond character to the bond between carbon of benzene nucleus and nitrogen of the \[N{{O}_{2}}\] group with the result the \[N{{O}_{2}}\] group is firmly bonded to the ring and therefore cannot be replaced other groups, i.e., it is very inert.
(ii) Displacement of the \[N{{O}_{2}}\] group : Although \[N{{O}_{2}}\] group of nitrobenzene cannot be replaced by other groups, but if a second \[N{{O}_{2}}\] group is present on the benzene ring of nitrobenzene in the \[o-\] or \[p-\]position, it can be replaced by a nucleophile. For example,

(iii) Reduction : Aromatic nitro compounds can be reduced to a variety of product as shown below in the case of nitrobenzene.
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\to \underset{\text{Nitrosobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}NO}}\,\to \underset{\text{Phenylhydroxylamine}}{\mathop{{{C}_{6}}{{H}_{5}}NHOH}}\,\to \underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
The nature of the final product depends mainly on the nature (acidic, basic or neutral) of the reduction medium and the nature of the reducing agent.
(a) Reduction in acidic medium

Reduction of dinitrobenzene with ammonium sulphide reduces only one \[N{{O}_{2}}\] group (selective reduction)

(b) Reduction in neutral medium :
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,+2H\underset{(-{{H}_{2}}O)}{\mathop{\xrightarrow{Zn\,dust\,+N{{H}_{4}}Cl}}}\,\underset{\begin{smallmatrix}
\text{Nitrosobenzene} \\ \text{(intermediate)} \end{smallmatrix}}{\mathop{{{C}_{6}}{{H}_{5}}NO}}\,\to \underset{\text{Phenylhydroxylamine}}{\mathop{{{C}_{6}}{{H}_{5}}NHOH}}\,\]
(c) Reduction in alkaline medium :
\[\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\xrightarrow{2[H]}\underset{\text{Phenyl hydroxylamine}}{\mathop{\left[ \begin{matrix} \underset{\text{Nitroso benzene}}{\mathop{{{C}_{6}}{{H}_{5}}NO}}\, \\ {{\text{C}}_{\text{6}}}{{H}_{5}}NHOH \\ \end{matrix} \right]}}\,\xrightarrow[-{{H}_{2}}O]{}\underset{\text{Azoxy benzene}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}- N\,\,\,\,\,\,\,\,\,}{\mathop{\underset{\,\,\,\,\,||}{\mathop{{{C}_{6}}{{H}_{5}}-N\to O}}\,}}\,}}\,\]
Azoxybenzene on further reduction yields azobenzene and hydrazobenzene.
\[\underset{\text{Azoxybenzene}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}\,- \,N\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}\underset{|\,|}{\mathop{N}}\,\to O}}\,}}\,\xrightarrow{2[H]}\underset{\text{Azobenzene}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}\,-\,N}{\mathop{{{C}_{6}}{{H}_{5}}\underset{|\,|\,}{\mathop{N}}\,}}\,}}\,\xrightarrow{2[H]}\underset{\text{Hydrazobenzene}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}\,- \,NH}{\mathop{{{C}_{6}}{{H}_{5}}\underset{|\,}{\mathop{N}}\,H}}\,}}\,\]
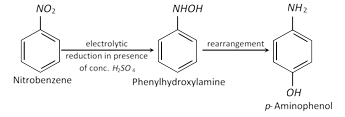
(d) Electrolytic reduction :
(iv) Electrophilic substitution : Since \[N{{O}_{2}}\] group is deactivating and m-directing, electrophilic substitution (halogenation, nitration and sulphonation) in simple aromatic nitro compounds (e.g. nitrobenzene) is very difficult as compared to that in benzene. Hence vigorous reaction conditions are used for such reaction and the new group enters the m-position.

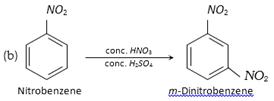
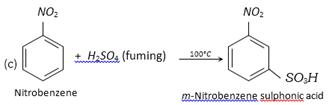
Although nitrobenzene, itself undergoes electrophilic substitution under drastic conditions, nitrobenzene having activating groups like alkyl, \[OR,N{{H}_{2}}\] etc. undergoes these reactions relatively more readily.
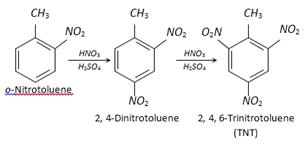
Sym-trinitrobenzene (TNB) is preferentially prepared from easily obtainable TNT rather than the direct nitration of benzene which even under drastic conditions of nitration gives poor yields.
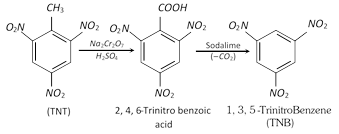
(v) Nucleophilic Substitution : Benzene is inert to nucleophiles, but the presence of \[N{{O}_{2}}\] group in the benzene ring activates the latter in \[o-\] and \[p-\]positions to nucleophiles.
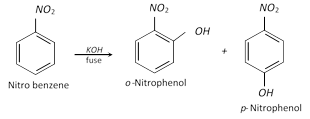
(vi) Effect of the \[\mathbf{-N}{{\mathbf{O}}_{\mathbf{2}}}\] group on other nuclear substituents
(a) Effect on nuclear halogen : The nuclear halogen is ordinarily inert, but if it carries one or more electron-withdrawing groups (like \[-N{{O}_{2}}\]) in \[o-\] or \[p-\]position, the halogen atom becomes active for nucleophilic substitutions and hence can be easily replaced by nucleophiles \[\left( KOH,N{{H}_{3}},NaO{{C}_{2}}{{H}_{5}} \right)\].

(b) Effect on phenolic \[-OH\] group : The acidity of the phenolic hydroxyl group is markedly increased by the presence of \[-N{{O}_{2}}\] group in \[o-\] and \[p-\] position.
The decreasing order of the acidity of nitrophenols follows following order
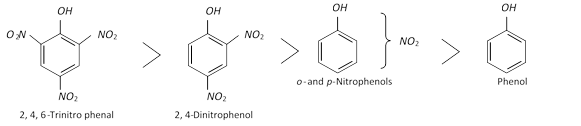
Increased acidity of \[o-\]and \[p-\]nitrophenols is because of the fact that the presence of electron-withdrawing \[-N{{O}_{2}}\] group in \[o-\]and \[p-\]position (s) to phenolic \[-OH\] group stabilises the phenoxide ions (recall that acidic nature of phenols is explained by resonance stabilisation of the phenoxide ion) to a greater extent.
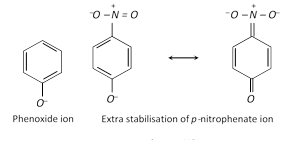
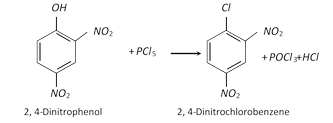
Due to increased acidity of nitrophenols, the latter react with phosphorus pentachloride to give good yields of the corresponding chloro derivative, while phenol itself when treated with \[PC{{l}_{5}}\] gives poor yield of chlorobenzene.
(4) Uses
(i) On account of their high polarity, aromatic nitro compounds are used as solvents.
(ii) Nitro compounds like TNT, picric acid, TNB etc. are widely used as explosives.
(iii) These are used for the synthesis of aromatic amino compounds.
(iv) Nitro benzene is used in the preparation of shoe polish and scenting of cheap soaps.
You need to login to perform this action.
You will be redirected in
3 sec
